Cell suspension culture A Suspension culture is a

























































































- Slides: 134

Cell suspension culture


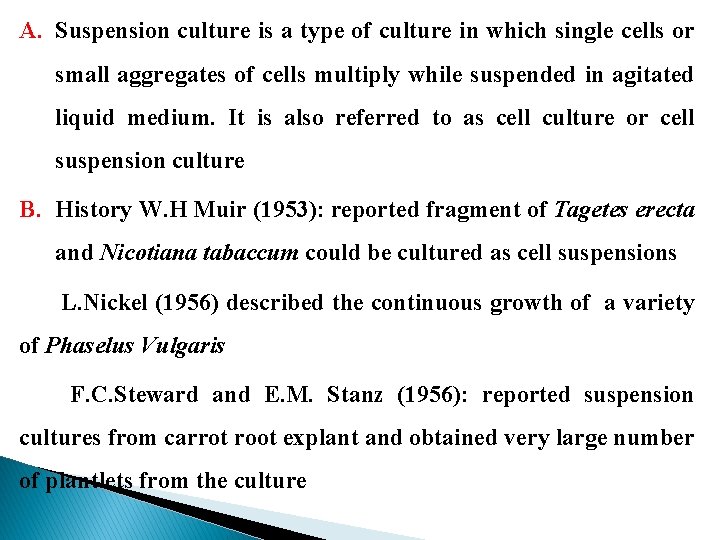
A. Suspension culture is a type of culture in which single cells or small aggregates of cells multiply while suspended in agitated liquid medium. It is also referred to as cell culture or cell suspension culture B. History W. H Muir (1953): reported fragment of Tagetes erecta and Nicotiana tabaccum could be cultured as cell suspensions L. Nickel (1956) described the continuous growth of a variety of Phaselus Vulgaris F. C. Steward and E. M. Stanz (1956): reported suspension cultures from carrot root explant and obtained very large number of plantlets from the culture

A. Callus grows as a mass--- difficult --- to study cellular events during its growth development--- chemically defined liquid media will free cells can study

A. Cell-suspension cultures • When friable callus is placed into the appropriate liquid medium and agitated, single cells and/or small clumps of cells are released into the medium and continue to grow and divide, producing a cell-suspension culture. This callus breaks up and readily disperses • The inoculum used to initiate cell suspension culture should neither be too small to affect cells numbers nor too large too allow the build up of toxic products or stressed cells to lethal levels. Separation of cells following cell division. Good suspension : Culture consisting of a high percentage of single cells and small clusters of cells. Different requirements for different cases. The choice of Suitable conditions is largely determined by trial and error.

Cell suspension culture • When callus pieces are agitated in a liquid medium, they tend to break up. • Suspensions are much easier to bulk up than callus since there is no manual transfer or solid support. • Large scale (50, 000 l) commercial fermentations for Shikonin and Berberine.




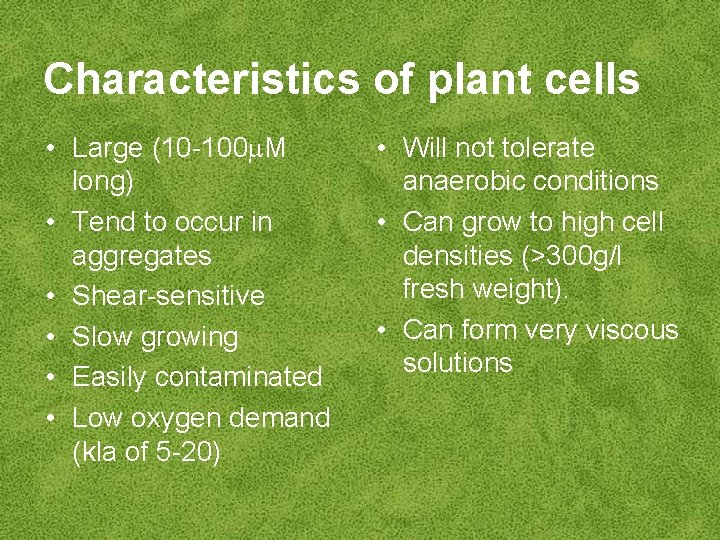
Characteristics of plant cells • Large (10 -100 M long) • Tend to occur in aggregates • Shear-sensitive • Slow growing • Easily contaminated • Low oxygen demand (kla of 5 -20) • Will not tolerate anaerobic conditions • Can grow to high cell densities (>300 g/l fresh weight). • Can form very viscous solutions

� Different time periods for subculturing � Some plants max. cell density is reached within about 18 -25 days. � In some plants as short as 6 -9 days � Nylon net or stainless steel filter is used to remove larger cell aggregates. � Small portion is withdrawn and cell density will be checked � 9 -15× 103 cells / cm 3 for sycamore. � The best speed for 100 -120 rpm � Liquid should be filled 20% of the size of the flask for adequate aeration.

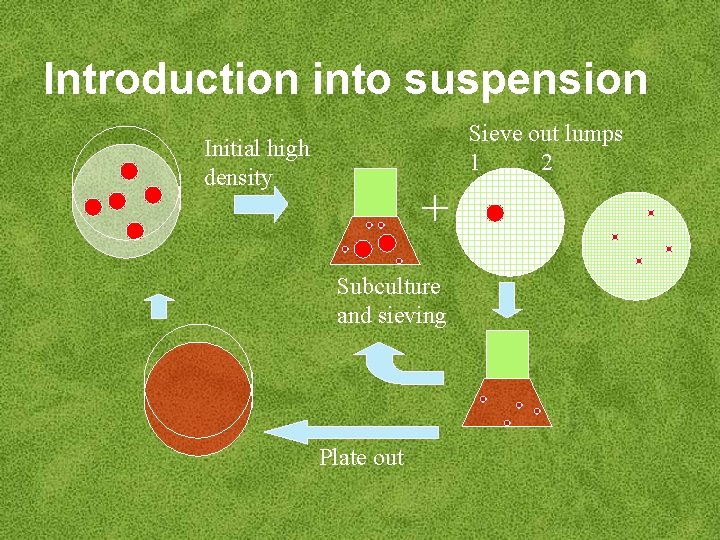
Introduction of callus into suspension • ‘Friable’ callus goes easily into suspension • Removal of large cell aggregates by sieving – 2, 4 -D • Plating of single cells – low cytokinin and small cell – semi-solid medium aggregates - only – enzymic digestion with viable cells will grow pectinase and can be re– blending introduced into suspension

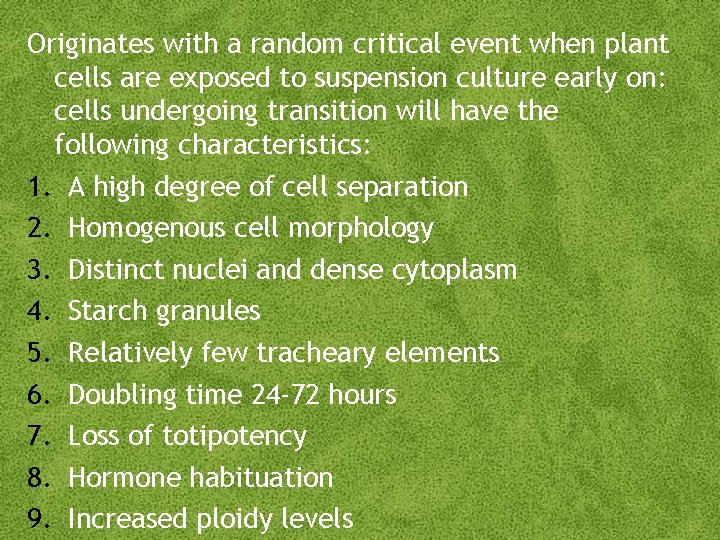
Originates with a random critical event when plant cells are exposed to suspension culture early on: cells undergoing transition will have the following characteristics: 1. A high degree of cell separation 2. Homogenous cell morphology 3. Distinct nuclei and dense cytoplasm 4. Starch granules 5. Relatively few tracheary elements 6. Doubling time 24 -72 hours 7. Loss of totipotency 8. Hormone habituation 9. Increased ploidy levels

Cell suspension culture Big pieces eliminated and after 2 -3 weeks only singe cells or cell aggregates transferred to new media. Suspension can be propagated by subculturing Ideally single cell cultures are desirable but hard to achieve. But can be achieved if the process of cell division, enlargement and differentiation is synchronized

Cell suspension culture Suspension culture eliminates many disadvantages ascribed to callus culture. Shaking of cells assures homogenous gaseous exchange, equal nutrient distribution (eliminates nutrient gradient within the medium and within the medium), removes polarity due to gravity

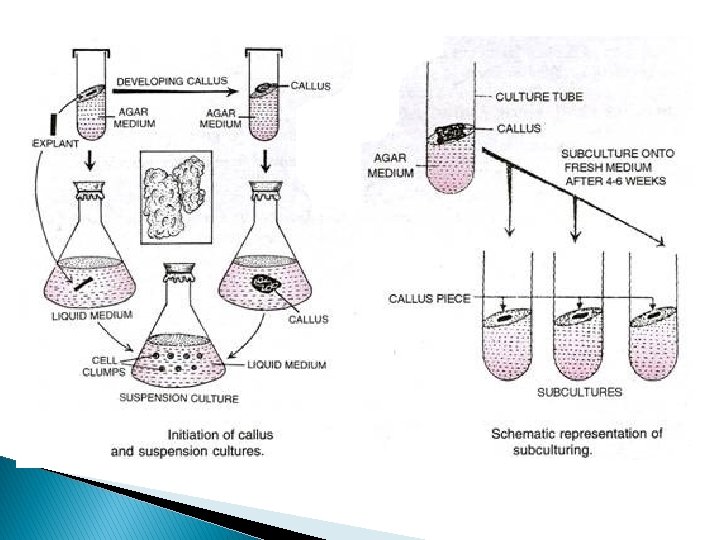
Cell suspension culture Can be initiated from pre-established callus (large amounts required 2 -3 g for 100 cm 3) culture or the explant (takes longer time to establish) on a rotary shaker. This provides aeration and nutrients and encourages the callus tissue to break up. Friable callus preferred but non friable callus can also be used by changing the media conditions Auxins and small amounts of cellulose and pectinase may be helpful

Cell suspension culture Initiation phase: development of suspension culture from the callus culture in shaking conical falsks Then passed through a nylon mesh to remove bigger aggregates

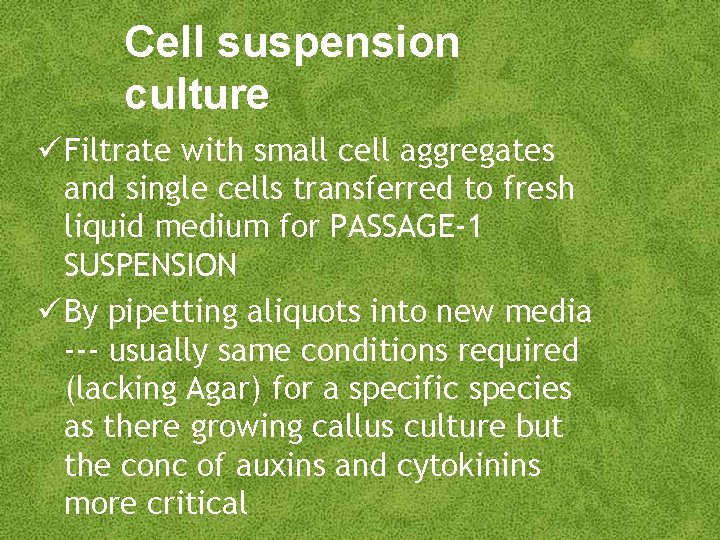
Cell suspension culture Filtrate with small cell aggregates and single cells transferred to fresh liquid medium for PASSAGE-1 SUSPENSION By pipetting aliquots into new media --- usually same conditions required (lacking Agar) for a specific species as there growing callus culture but the conc of auxins and cytokinins more critical

Cell suspension culture Cells have different microenvironment than free floating cells 1. Have different shapes oval round, elongates, coiled etc 2. Usually entirely thin walled with few exceptions that arise as aggregates 3. Suspension cultures may form the whole plant or embryoids depending upon the species

Cell suspension culture (passage time can be learned through experience usually cells 18 -25 days for full density, very active cultures may take 6 -9 days)

Cell suspension culture Two types of suspension culture 1. BATCH CULTURE: Cells grow in finite volume of agitated liquid eg. 20 -60 ml in conical flasks incubated on orbital platform shakers at speed of 0 -120 rpm. Same repeated for subsequent transfers

Cell suspension culture Slow rotating cultures: grown in nipple flasks. 8 nipples– can rotate– each sample bathes and then is exposed to air at, rotates at 1 -2 rpm Shake culture: 60 -180 rpm– conical flasks circular motion Spinning cultures: 10 l bottles, rpm 120 at an angle of 450 in a culture spinner Stirred culture: sample inside a culture medium displaced by bubbling air. Magnatic stirrer (20600 rpm) can be used to agitate culture (1. 5 -10 liters).

Cell suspension culture CONTINUOUS CULTURE SYSTEM: medium continuously replaced; replaced when certain nutrient has been depleted; cell always remain in steady state of active growth phase Chemostats: circular or cylindrical; have inlets and outlets pores for aeration and introduction and removal of cells and medium; media stirred by a magnetic stirrer. Media introduced –equivalent to media dispensed to compensate for the cells lost during media removal by new cell division. Can be always maintained at a steady state– thus density, growth rate, chemical composition and metabolic activity of cells remain same. Ideal for studying growth kinetics

Cell suspension culture Turbidostats: can maintain turbidity and p. H of the medium due to a monitor system that detects these levels and adds new media and/or takes out old media.

Introduction into suspension Sieve out lumps 1 2 Initial high density + Subculture and sieving Plate out

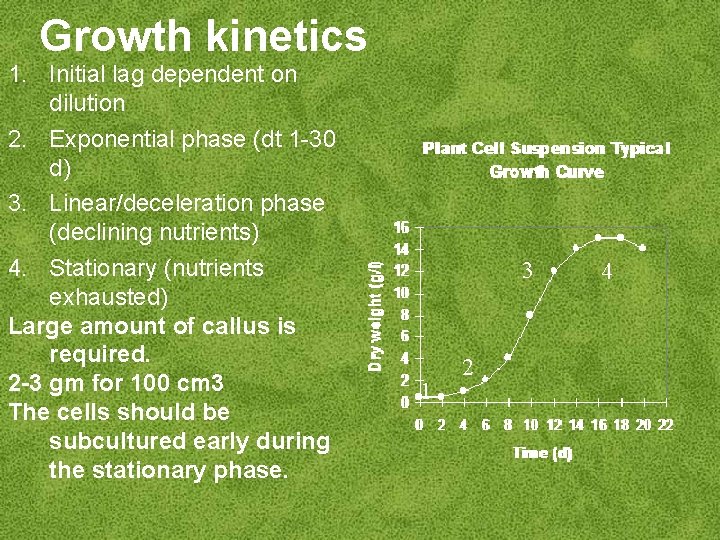
Growth kinetics 1. Initial lag dependent on dilution 2. Exponential phase (dt 1 -30 d) 3. Linear/deceleration phase (declining nutrients) 4. Stationary (nutrients exhausted) Large amount of callus is required. 2 -3 gm for 100 cm 3 The cells should be subcultured early during the stationary phase. 3 1 2 4

Growth kinetics Lag phase cells adjust and get ready for cell division Log phase cells divide Linear phase: Linear increase in number Stationary phase: some cells start to die a plateau Low or high initial density will ot allow the cells to grow; Critical initial density CID: cell density required for cell division (usually 9 -15 X 103) Growth can be monitored by looking at the cells under a microscope or in haemocytometer

Test for viability Flourescien diacetate stain– dead cells would fluoresces red Evans blue: in viable blue viable unstained

Reactors for plant suspension cultures • • • Modified stirred tank Air-lift Air loop Bubble column Rotating drum reactor

Synchronization • Cold treatment: 4 o. C • Starvation: deprivation of an essential growth compound, e. g. N →accumulation in G 1 • Use of DNA synthesis inhibitors: thymidine, 5 -fluorodeoxyuridine, hydroxyurea • Colchicine method: arresting the cells in metaphase stage, measured in terms of mitotic index (% cells in the mitotic phase)

Ways to increase product formation • Select • Start off with a producing part • Modify media for growth and product formation. • Feed precursors or feed intermediates (bioconversion) • Produce ‘plant-like’ conditions (immobilisation)

Selection Strategies • • Positive Negative Visual Analytical Screening

Positive selection • Add into medium a toxic compound e. g. hydroxy proline, kanamycin • Only those cells able to grow in the presence of the selective agent give colonies • Plate out and pick off growing colonies. • Possible to select one colony from millions of plated cells in a days work. • Need a strong selection pressure - get escapes

Negative selection • Add in an agent that kills dividing cells e. g. chlorate / BUd. R. • Plate out leave for a suitable time, wash out agent then put on growth medium. • All cells growing on selective agent will die leaving only non-growing cells to now grow. • Useful for selecting auxotrophs.

Visual selection • Only useful for colored or fluorescent compounds e. g. shikonin, berberine, some alkaloids • Plate out at about 50, 000 cells per plate • Pick off colored / fluorescent-expressing compounds (cell compounds? ) • Possible to screen about 1, 000 cells in a days work.

Analytical Screening • Cut each piece of callus in half • One half subcultured • Other half extracted and amount of compound determined analytically (HPLC/ GCMS/ ELISA)

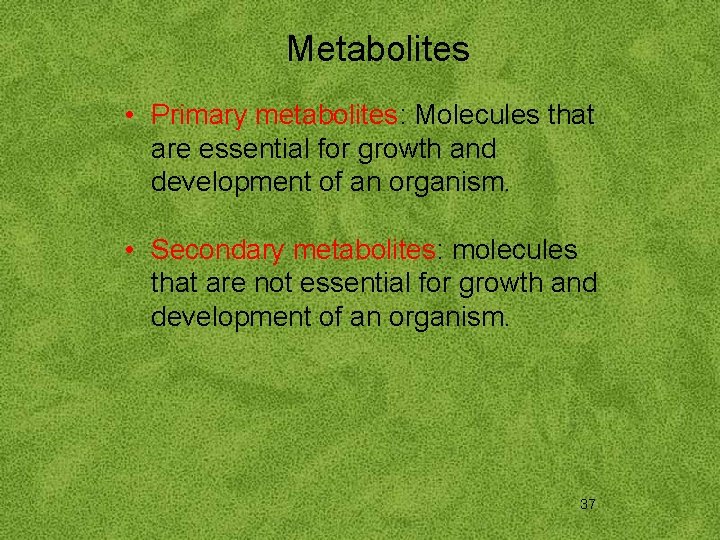
Metabolites • Primary metabolites: Molecules that are essential for growth and development of an organism. • Secondary metabolites: molecules that are not essential for growth and development of an organism. 37

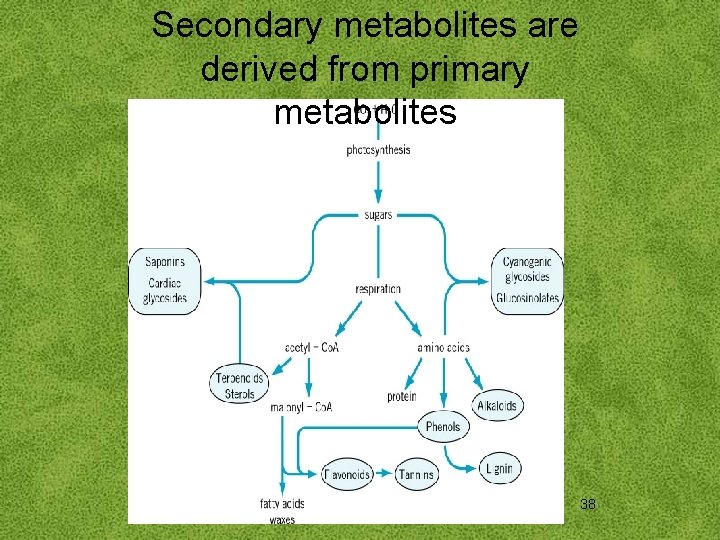
Secondary metabolites are derived from primary metabolites 38

Why secondary metabolites? • Chemical warfare to protect plants from the attacks by predators, pathogens, or competitors • Attract pollinators or seed dispersal agents • Important for abiotic stresses • Medicine • Industrial additives 39

Secondary metabolites • Possibly over 250, 000 secondary metabolites in plants • Classified based on common biosynthetic pathways where a chemical is derived. • Four major classes: Alkaloids, glycosides, phenolics, terpenoids 40

Alkaloids • Most are derived from a few common amino acids (i. e. , tyrosine, tryptophan, ornithine or argenine, and lysine) • Compounds have a ring structure and a nitrogen residue. • Indole alkaloids is the largest group in this family, derived from tryptophan • Widely used as medicine 41

Phenolics • Derived from aromatic amino acids, such as phenylalanine, tyrosin, and trytophan. • All contain structures derived from phenol • Some examples: Coumarins: antimicrobial agents, feeding deterrents, and germination inhibitors. Lignin: abundant in secondary cell wall, rigid and resistant to extraction or many degradation reagents. 42

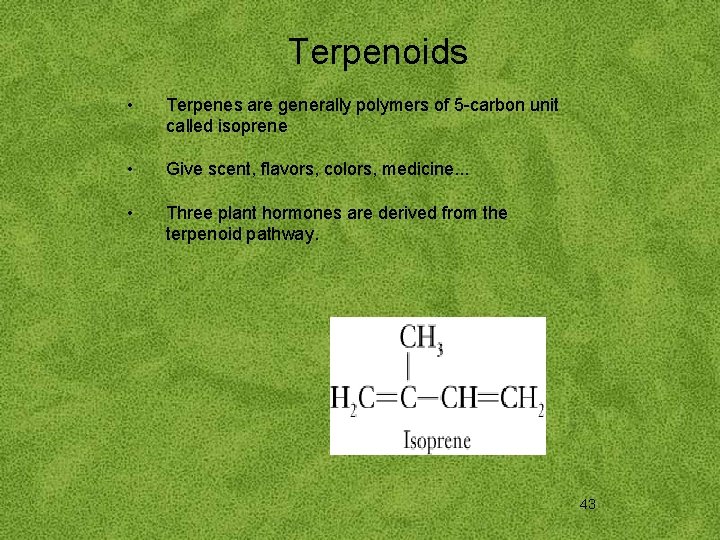
Terpenoids • Terpenes are generally polymers of 5 -carbon unit called isoprene • Give scent, flavors, colors, medicine. . . • Three plant hormones are derived from the terpenoid pathway. 43

Glycosides • Compounds that contain a carbonhydrate and a noncarbohydrate • Glucosinolates: found primarily in the mustard family to give the pungent taste. 44

Taxol Taxus brevifolia Nutt. • Taxol is a terpenoid • "the best anti-cancer agent” by National Cancer Institute • Has remarkable activity against advanced ovarian and breast cancer, and has been approved for 45 clinical use.

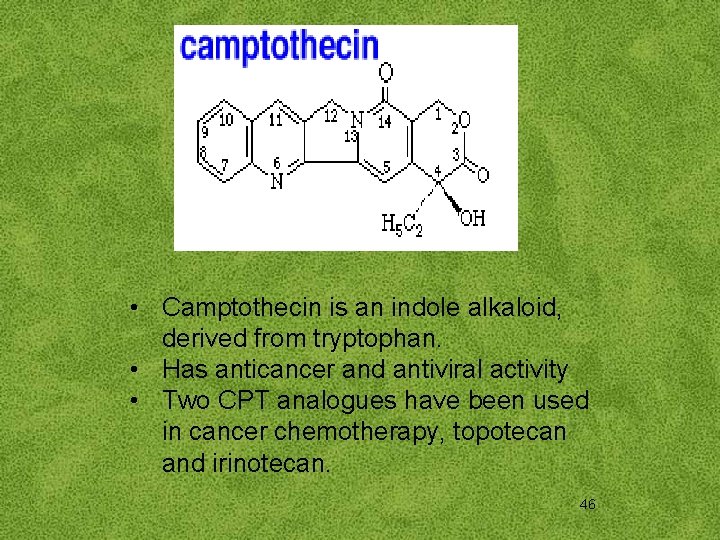
• Camptothecin is an indole alkaloid, derived from tryptophan. • Has anticancer and antiviral activity • Two CPT analogues have been used in cancer chemotherapy, topotecan and irinotecan. 46

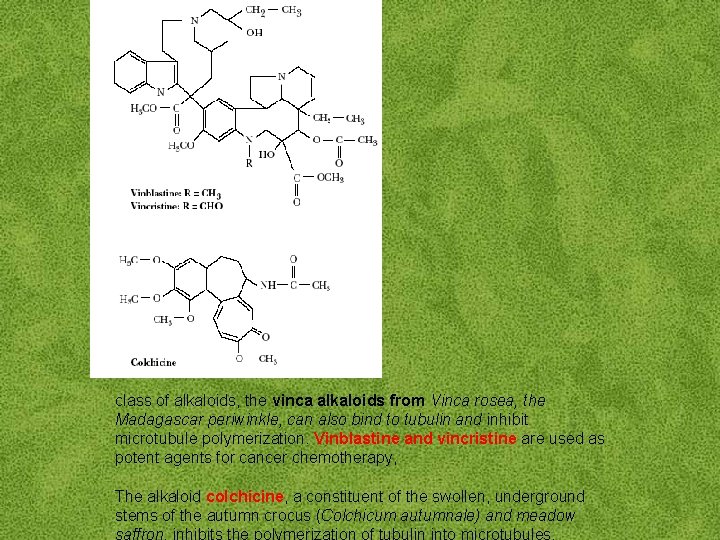
class of alkaloids, the vinca alkaloids from Vinca rosea, the Madagascar periwinkle, can also bind to tubulin and inhibit microtubule polymerization. Vinblastine and vincristine are used as potent agents for cancer chemotherapy, The alkaloid colchicine, a constituent of the swollen, underground stems of the autumn crocus (Colchicum autumnale) and meadow

Importance Can help study: cell physiology, biochemistry, metabolic events at the level of single cells or aggregates. Help understand the process of organ and embryoid formation Single cell clones Secondary metabolites Mutagenesis to form mutant cell clones. Mutagens such EMS can be directly added to the medium Cell suspension culture techniques are very important for plant biotransformation and plant genetic engineering. Single cells can be placed onto agar medium formation of callus and thus forming a whole plant from a single cell

PATHOGEN ELIMINATION Most plants --- systemic diseases caused by fungi, viruses bacteria nematodes and mycoplasmas • Can lead to death • Also reduction in yield and quality of crops • Pathogens nearly always transferred by vegetative propagation • Viruses not only in vegetative propagated organs but also all seeds

PATHOGEN ELIMINATION q Pathogen elimination desirable: • Better yield • Trade across international boundaries q Can treat fungi with fungicides and bacteria with bactericides but no treatment for viruses among plants q Antimetabolites have helped eradicate viruses from culture media for lily and apple

PATHOGEN ELIMINATION q To eradicate viruses one can select for heathy plants and propagate them q For entire population infected with viruses tissue culture is required

PATHOGEN ELIMINATION q Apical meristem has no or the least amount of viruses; as tissues grows older and moves away from meristem the viral titre also increases. This may be due to • Viruses move through vascular system it is absent in the meristem • High metabolic activity in meristem reduces viral replication • High endogenous auxin level may reduce viral multiplication • Due to this gradient of viral titer scientists grew meristem and obtained from it disease free plants • Meristem culture also frees from other pathogens q Plants are free of viruses whose test have been developed q Well only be virus free if it is free from all known viruses

METHODS FOR VIRAL ELIMINATION q HEAT TREATMENT • Before meristem culture viral eradication was by heat treatment (thermotherapy) • Through hot water (dormant buds) or air (growing shoots) can inactivate viruses without any harm to the host • Better host survival in hot air treatment

HOT AIR TREATMENT • IN A ATHERMOTHERAPY CHAMBER AT 35 -40 C from a few hours to months • Requires adequate humidity and light • Adequate carbohydrates which can be achieved by pruning • Temperature should be raised gradually over the course of a few days • Small cuttings taken and grafted onto healthy rootstocks • Drawbacks: not for all viruses; can be counter productive by increasing the sensitivity of cured plants to pathogens over prolonged periods of treatment • If some viruses escape this treatment they can be eradicated by meristem tip culture

MERISTEM TIP-CULTURE q Size of explant important; if it is too big then it can have viruses’ q Also in some cases host virus combination also important q Same aseptic techniques employed as other cultures q Meristem tips can be isolated from apices of stems, tuber sprout, leaf axils, buds of cuttings or germinated seeds q My not need surface sterilization as they are protected very well by leaf primordia; but as a precautionary measure should be dipped in 75% alcohol q Surface sterilization required for underground plant organs

MERISTEM TIP-CULTURE q As explant is very small a stereoscopic microscope should be used and care should be taken to not desiccate the meristem q Can perform this excision over a petri-dish lined with sterile filter paper q Instruments need to be sterilized

MERISTEM TIP-CULTURE Method 1. Hold bud under microscope with a forcep 2. Leaves and leaf primordia are removed by needle to expose apical meristem (A shiny dome) 3. Excise meristem by a clean cut with a sharp blade 4. Same instruments can be used to transfer the tip to culture media

MERISTEM TIP-CULTURE Physiological condition of the explant: • Should take actively growing bud; so position from where taken important. In some cases terminal buds better than auxiliary buds in other cases no difference. • Season or time of year also important; should be taken when active not dormant or after dormancy has been broken with suitable treatment • More axillary buds can be used to get better escape from viral titer • Should also be able to root and shoot so need to keep those requirements in mind

MERISTEM TIP-CULTURE AND THERMOTHERAPY • Some viruses present in the meristem such as TMV; both treatments effective in getting rid of viruses under such circumstances • Heat treatment can be given to mother plant as well as the meristem culture • Can alternate heat and normal temperatures through out the day to avoid damage to the explant or deterioration of meristem tip culture

Culture media • Small amounts of auxins and cytokinins have been useful in the MS media • GA 3 in some cases can also be added • Both agar and liquid media have been used but agar mostly preferred • In case of liquid media the explant can be placed on a filter paper dipped at both sides into the media and the explant resting above the media

STORAGE CONDITIONS • Need adequate light except those cultures that need to be grown in the dark to minimize production of polyphenols • Light can be increased as the explant grows after a suitable period of time

CHEMICAL TREATMENT • Not very useful and shows conflicting results • Antimetabolites had little effect (malachite green) • Nucleoside analogue ribavirin removed PXC virus in potato, CMV and alfalfa mosaic virus in other cultures • Vidarbine, cycloheximide and actinomycin D have aslo been useful

OTHER METHODS (in vivo) • In callus cultures distribution of viruses is uneven; some cells don’s have them • This is because: 1. Virus replication cannot keep pace with the dividing cells 2. Cells acquire resistance to viruses through mutagenesis 3. Some drawbacks include: 1: Genetic instability and 2: low potential of plant regeneration 4. Other methods include somatic cell hybridization, gene transformation and somaclonal variations

VIRUS INDEXING • Used to determine if viruses really have been eradicated; need to do several times during the plant growth period as some viruses may sprout later. A consistent negative result will only show that viruses are not present:

VIRUS INDEXING: METHODS USED • Sap transmission test: Most sensitive of all three methods Leaves taken and ground in a pestle and mortar with equal volume of buffer. Then sprayed on an indicator plant (Plant susceptible to the virus), if plant develops symptoms then virus present • Serology: a drop of sap from the test plant can be added an antiserum solution obtained from rabbits with the specific antibodies; precipitation indicated a reaction between the viral proteins and the antibodies (ELISA) the enzyme linked immunosorbant assay can be used for this purpose • EM STUDIES:

MICROGRAFTING • Initiating meristem cultures from woody plants is very difficult • So can do micro grafting • Can take meristem and graft it onto a virus free rootstock (seedling) maintained and propagated in vitro. • Specially useful in eliminating viruses from fruit crops

Eradicating other pathogens • Fungi, bacteria and mycoplasmas can also be removed by using meristem-tip culture and callus culture

PROTOPLAST CULTURE

1. Introduction The term protoplast was introduced by Hanstein in 1880. It refers to the cellular content excluding cell wall or can also be called as naked plant cell. It is described as living matter enclosed by a plant cell membrane. Protoplast isolation for the first time was carried out by Klercker in 1892 using mechanical method on the plasmolysed cells. The application of protoplast technology for the improvement of plants offers fascinating option to complement conventional breeding programs. The ability of isolated protoplasts to undergo fusion and take up macromolecules and cell organelles offers many possibilities in genetic engineering and crop improvement. 69

The experiments involving protoplasts consist of three stages – i. protoplast isolation ii. protoplast fusion (leading to gene uptake) iii. development of regenerated fertile plants from the fusion product (Hybrid). Depending upon the species and culture conditions, the protoplasts may have the potential to: i. regenerate a cell wall ii. dedifferentiate to form callus iii. divide mitotically and proliferate clonally iv redifferentiate into shoots, roots or embryos 70 and produce a complete plantlet.

However, to fully explore the potentials for protoplast-technology, efficient and reproducible methods for protoplast isolation and purification must first be established. Since leaf tissue is a readily accessible source of genetically uniform cells, it is often desirable to use mesophyll protoplasts in somatic hybridization studies, but, leaf tissues, in general, do not yield large number of protoplasts owing to the difficulty in removing the lower epidermis. An alternative, therefore, is the cultured cell material where protoplasts can show greater potential to divide. 71

2. Protoplast isolation may be carried out by 1. Mechanical disruption method or 2. Enzymatic method. * NOTE: Out of these two methods, enzymatic method is preferred as it provides better protoplast yield with low tissue damage while mechanical method causes maximum tissue chopping with lower protoplast yields. 72

i. Mechanical method Klercker in 1892 pioneered the isolation of protoplasts by mechanical methods. In this method, the cells were kept in suitable plasmolyticum, for example CPW containing 13% w/v mannitol. Once the plasmolysis is complete, while remaining in the osmoticum, the leaf lamina would be cut with a sharp-edged knife. In this process some of the plasmolyzed cells were cut only through the cell wall, releasing intact protoplasts while some of the protoplasts may be damaged inside many cells. Protoplasts that were trapped in a cell and only the corner had been cut off could be encouraged to come out by reducing the osmolarity slightly to force the protoplasts swell to force their way out of the cut surface. The released protoplasts then have to be separated from damaged ones and cell debris. 73

Disadvantages • Lower protoplast yield. • Labour intensive method. • Protoplast obtained has low viability. • Method is applicable only to vacuolated cells. 74

ii. Enzymatic method In 1960, E. C. Cocking demonstrated the possibility of enzymatic isolation of a large number of protoplasts from cells of higher plants. This method involves leaf sterilization followed by peeling of the lower epidermis to release cells which are plasmolyzed and added to enzyme mixture followed by harvest of protoplast as shown in (Figure). Either of the procedures for enzymatic isolation can be used: sequential enzymatic hydrolysis 75 or mixed enzymatic hydrolysis.

A. SEQUENTIAL ISOLATION In the sequential isolation, 1. Firstly, cells are separated by the use of a maceration enzyme – a pectin hydrolyzing enzyme such as, macerozyme or Pectolyase. 2. Once the cells are separated, they are washed in CPW solution free of enzymes but containing plasmolyticum by gentle centrifugation (100 g). 3. The pellet is retained and resuspended in the second enzyme like, cellulases and hemicellulases, used to hydrolyse the remaining cell was component. 76 4. Once the protoplasts are released they are washed with CPW to remove the debris.

B. MIXED ENZYMATIC APPROACH In the mixed enzymatic approach, Plant tissues are plasmolyzed in the presence of a mixture of pectinases and cellulases, thus, inducing simultaneous separation of cells and degradation of their walls to release the protoplasts directly in a single step. 77

Figure : Steps involved in protoplast isolation, fusion and regeneration 78

CONDITIONS REQUIRED FOR ENZYME ACTIVITY Enzymes are p. H and temperature dependent, thus, for enzymatic release of protoplast an enzyme showing activity at p. H range 4. 7 -6. 0 and temperature range of 25 -30°C is used. • Duration of enzyme pretreatment and condition of light presence required for incubation may also be determined. • Enzyme mixture used should essentially consist of cellulase, hemicellulase and pectinase which facilitate the degradation of cellulose, hemicelluloses and pectin, respectively. • The concentration of sugar alcohols used 79 as osmoticum (mannitol) must be empirically defined.

Factors affecting yield and viability of protoplasts 1. Source of material 2. Pre-enzyme treatments 3. Enzyme treatment 80

1. SOURCE OF MATERIAL Leaves were the most convenient source of the plant protoplasts because it allows 1. The isolation of a large number of relatively uniform cells without killing the plants. 2. Moreover the mesophyll cells are loosely arranged, the enzymes have an easy access to the cell wall. 3. The parent plant age and the conditions in which it is growing have profound effect on the yield of protoplast. 4. Due to the difficulty in isolating culturable protoplast from leaf cells of cereals and some other species their cultured cells can be used as a source material. 5. The yield of protoplasts depends upon the growth rate and growth phase of the cells. 6. Generally embryogenic suspension cultures are used to obtain totipotent protoplasts. 81

2. PRE-ENZYME TREATMENTS To facilitate the penetration of enzyme solution into the intercellular spaces of leaf, which is essential for effective digestion, various methods are followed. 1. The most commonly used method is to peel the lower epidermis and float the stripped pieces of leaf on the enzyme solution in a manner that the peeled surface is in a contact with the solution. 2. Most of the time it is not convenient to peel the epidermis, in such cases cutting the leaf or tissue into small strips (1 - 2 mm wide) has been found useful. When combined with vacuum infiltration the latter approach has proved very effective. 4. Brushing of leaves with a soft brush or with the cutting edge of a scalpel may also improve the enzymatic action. 5. Large calli are chopped into pieces and can be transferred to enzyme mixture. 82 6. Agitation of incubation mixture during enzyme treatment improves protoplast yield from cultured cells.

3. ENZYME TREATMENT q The release of protoplast is very much dependent on the nature and concentration of the enzymes used. q The two major enzymes required for the isolation of protoplast are cellulase and pectinase. q The cellulase is required to digest the cellulosic cell walls and the pectinase mainly degrades the middle lamella. q Some of the tissues may require other enzymes like, hemicellulase, driselase, macerozyme and pectolyase. q The activity of enzyme is p. H dependent. The p. H of the enzyme solution is adjusted somewhere 83 between 4. 7 to 6. 0.

CHOICE OF PLASMOLYTICUM The two most commonly used compounds are the sugar alcohols - mannitol and sorbitol. Of these, mannitol is the most preferred since it is not metabolized by the plant cells. Once the protoplast divides and regenerates the cell wall, no more osmoticum is required. It is, therefore, should be removed gradually from the medium otherwise cell division stops. To slowly remove the osmoticum from the medium, the protoplast can be isolated in a high osmoticum mixture consisting of both mannitol and sucrose, the sucrose will be metabolized by the dividing protoplasts and thus, will reduce the osmolarity of the medium. Normally, mannitol is used 84 at concentration range of 11 -13%.

Cocking, Peberdy and White – CPW A solution into which the osmoticum is often, but not always, added is called CPW salts mix or CPW for short. This has been observed much more beneficial than using distilled water as a solvent in obtaining high yields of viable protoplasts. Although CPW is most widely used solution into which osmoticum or enzymes are added, some times culture medium used to grow cells or plants can also be utilized for protoplast isolation at one tenth concentration. Low concentration of culture medium is much more advantageous when compared with CPW. 85

Table: Salt mix of protoplast washing media solution (Cocking, Peberdy and White – CPW) 86

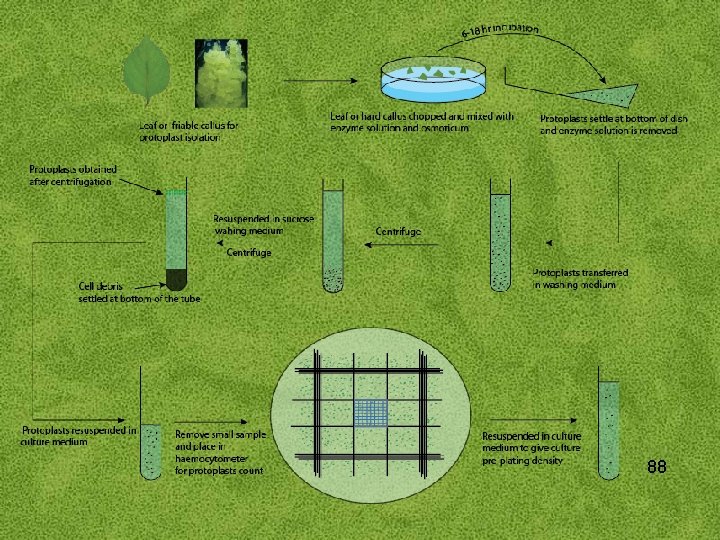
PROTOPLAST PURIFICATION Enzyme treatment results in suspension of protoplast, undigested tissues and cellular debris. This suspension is passed through a metal sieve or a nylon mesh (50 -100 µm) in order to remove undigested cellular clumps. The filtered protoplast-enzyme solution is mixed with a suitable volume of osmoticum, solution is centrifuged to pellet the protoplasts, pellet of protoplast is resuspended in osmoticum of similar concentration as used in enzyme mixture. The protoplast band is sucked in Pasteur pipette and is put into other centrifuge and finally suspended in culture 87 medium at particular density; this is explained by the Figure.

88

PROTOPLAST VIABILITY The isolated protoplast must have a spherical shape when observed by a light microscope, protoplast can be stained using following stain: 1. Fluorescein diacetate staining method: FDA accumulates inside the plasmalemma of viable protoplasts. Live protoplasts contain esterases which cleave FDA to release fluorescein which fluoresces yellowish-green using fluorescence microscopy within 5 min. FDA dissociates from membrane after about 15 min. It is used at a concentration of 0. 01% dissolved in acetone. 2. Calcofluor White staining: This staining method assures protoplast viability by detecting onset of cell wall formation. Calcofluor binds to beta linked glucosides in newly synthesized cell wall which can be observed as a fluorescent ring around the membrane. Optimum staining is achieved when 0. 1 ml of protoplast is mixed with 5. 0 μl of 0. 1% w/v solution of CFW. 3. Protoplast viability can also be detected by monitoring oxygen uptake of cells by oxygen electrode, which shows respiration. 4. Variation of protoplast size with changing osmotic concentration also enables viability of protoplast. 89

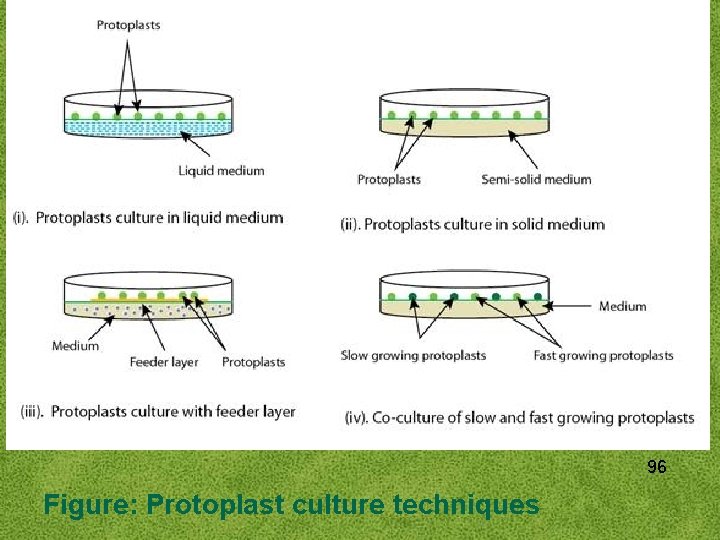
3. PROTOPLAST CULTURE Protoplasts culture techniques The culture requirements and the culture methods are same for both protoplasts and single cells. The main difference is the requirement of suitable osmoticum for protoplasts until they regenerate a strong wall. Isolated protoplasts are either cultured in liquid or semisolid agar or agarose media plates, sometimes the protoplast is first grown in liquid media and then transferred into the agar media plates. 90

The following techniques have been adopted in order to maintain number of protoplast population between minimum and maximum effective densities after plating up: i. Liquid method ii. Embedded in Agar/ Agarose iii. Feeder layer iv. Co-culturing 91

i. Liquid method This method is preferred in earlier stages of culture as it provides (a) easier dilution and transfer, (b) the osmotic pressure of liquid media can be effectively reduced after a few days of culture (c) the cell density can be reduced or cells of special interest can be isolated easily. In Liquid medium, the protoplast suspension is plated as a thin layer in petriplates, incubated as static culture in flasks or distributed in 50100 μl drops in petriplates and stored in a 92 humidifier chamber.

ii. Embedded in Agar/ Agarose is a preferred choice in place of agar and this has improved the culture response. This method of agar culture keeps protoplast in fixed position, thus, prevents it from forming clumps. Immobilized protoplasts give rise to clones which can then be transferred to other media. In practice, the protoplasts suspended in molten (40°C) agarose medium (1. 2% w/v agarose) are dispensed (4 ml) into small (3. 55 cm diameter) plates and allowed to solidify. The agarose layer is then cut into 4 equal sized blocks and transferred to larger dishes (9 cm diameter) containing liquid medium of otherwise the same composition. Alternatively, protoplasts in molten agarose medium are dispensed as droplets (50 -100 μl) on the bottom of petri plates and after solidification the droplets are submerged in the same liquid medium. 93

iii. Feeder layer In order to culture protoplast at low density, a feeder layer technique is adopted. A feeder layer of X-ray irradiated nondividing but metabolically active protoplasts after washing are plated in soft agar medium. Non-irradiated protoplasts of low density are plated over this feeder layer. The protoplasts of the same species or different species can be used as a feeder 94 layer.

iv. Co-culturing This method involves co-culture of protoplasts from two different species to promote their growth or that of the hybrid cells. Metabolically active and dividing protoplasts of two types - slow and fast growing are cultured together, the fast growing protoplast provide other species with diffusible chemicals and growth factors which helps in cell wall formation and cell division. The co-culture methods is generally used where calli arising from two types of protoplasts can be morphologically distinguished. For example, protoplasts isolated from albino plants and green plants are easily distinguishable based on color where albino protoplast will develop non 95 green colonies.

96 Figure: Protoplast culture techniques

CULTURE MEDIUM The nutritional requirement of protoplast is almost similar to that of the cultured plant cells. Mostly the salts of MS (Murashige and Skoog, 1962) and B 5 (Gamborg et al. 1968) media and their modifications have been used. Ammonium salts have been found detrimental to protoplasts survival of many species, and media have been devised that either have a reduced concentration of ammonium or lack it. Concentration of zinc is reduced while the concentration of calcium is increased as it enhances the membrane stability. Osmolarity is maintained by addition of sorbitol, mannitol, glucose or sucrose and mannitol being widely used osmoticum as it is not used by the dividing 97 cells, thus, maintains the osmolarity of the medium.

Glucose is preferred carbon source as sucrose do not satisfy protoplast culture. One or two amino acids are added at low concentration. Growth regulators are required essentially in protoplast culture generally high concentration of auxins (NAA, 2, 4 -D) along with lower concentration of cytokinins (BAP, Zeatin ) is used. Environmental conditions: High light intensity inhibits growth of protoplast hence initially protoplast is grown in dim light for few days and then transferred to light of about 20005000 lux. However, better results are obtained when cultured in darkness. 98

Plating density Like cell cultures, the initial plating density of protoplasts has profound effect on plating efficiency. Protoplasts are cultured at a density of 1 x 104 to 1 x 105 protoplasts ml-1 of the medium. At high density the cell colonies arising from individual protoplasts tend to grow into each other resulting into chimera tissue if the protoplast population is genetically heterogeneous. Cloning of individuals cells, which is desirable in somatic hybridization and mutagenic studies, can be achieved if protoplasts or cells derived from 99 them can be cultured at a low density.

4. Protoplast development and regeneration Protoplast starts to regenerate a cell wall within few days (2 -4 days) of culture and during this process, protoplasts lose their characteristic spherical shape which has been taken as an indication of new wall regeneration. Cell wall regeneration can be confirmed by Calcofluor White staining method. There is direct relationship between wall formation and cell division. Protoplasts which are not able to regenerate a proper wall fail to undergo normal mitosis. Protoplasts with a poorly developed wall often show budding and may enlarge several times their original volume. They may become multinucleate because karyokinesis is not accompanied by cytokinesis. Among other reasons, inadequate washing of the protoplasts prior to culture leads to these 100 abnormalities.

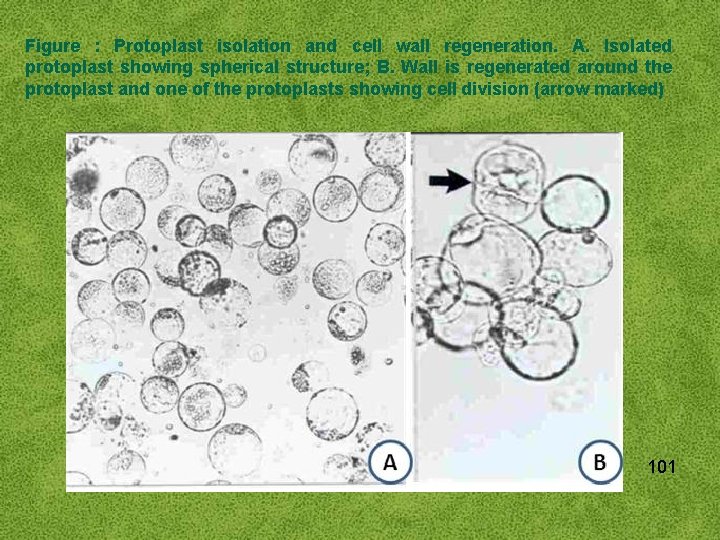
Figure : Protoplast isolation and cell wall regeneration. A. Isolated protoplast showing spherical structure; B. Wall is regenerated around the protoplast and one of the protoplasts showing cell division (arrow marked) 101

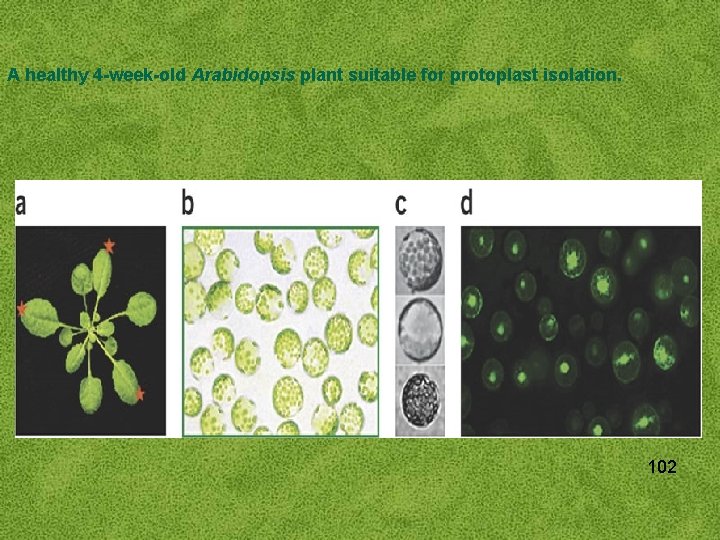
A healthy 4 -week-old Arabidopsis plant suitable for protoplast isolation. 102

103

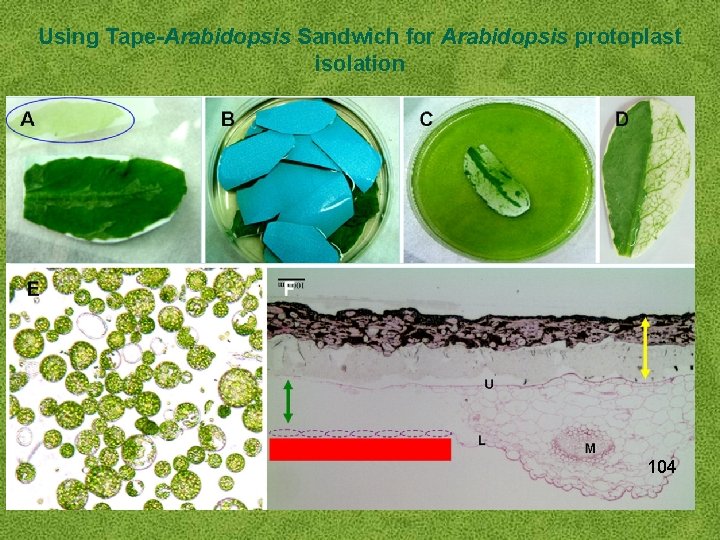
Using Tape-Arabidopsis Sandwich for Arabidopsis protoplast isolation 104

105

106

Figure 1. Isolation of protoplasts from Arabidopsis seedlings. A, Tissues from 14 d-old seedlings of Arabidopsis were collected and converted to protoplasts by a modified procedure of Chen and Halkier (2000). B, Protoplasts were purified by Suc density gradient centrifugation and collected at the interface of enzyme solution and W 5 buffer (white arrows). C, Bright-field microscopy of protoplasts. 107 Microphotographs were collected using a cooled CCD camera interfaced with the Zeiss Axioscope 2 plus microscope.

And completes process when provided with suitable condition of light, p. H and temperature newly synthesized protoplast can be visualized by staining. Once the cell wall formation is completed, cells undergo division resulting in increase size of cells. After an interval of 3 weeks, small cell colonies appear, these colonies are transferred to an osmotic-free callus induction medium. This is followed by introduction into organogenic or embryogenic medium leading 108 to plantlet development.

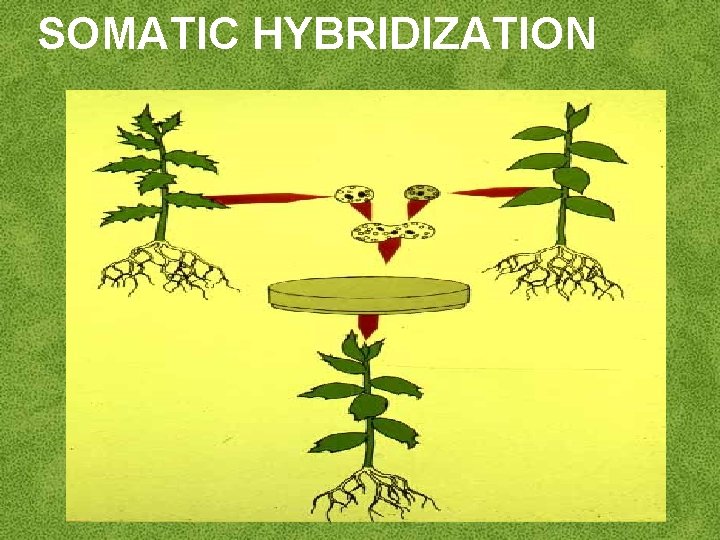
SOMATIC HYBRIDIZATION

Development of hybrid plants through the fusion of somatic protoplasts of two different plant species/varieties is called somatic hybridization

HISTORY The fusion of plant protoplasts is not a particularly new phenomenon. Kuster in 1909 described the process of random fusion in mechanically isolated protoplasts.

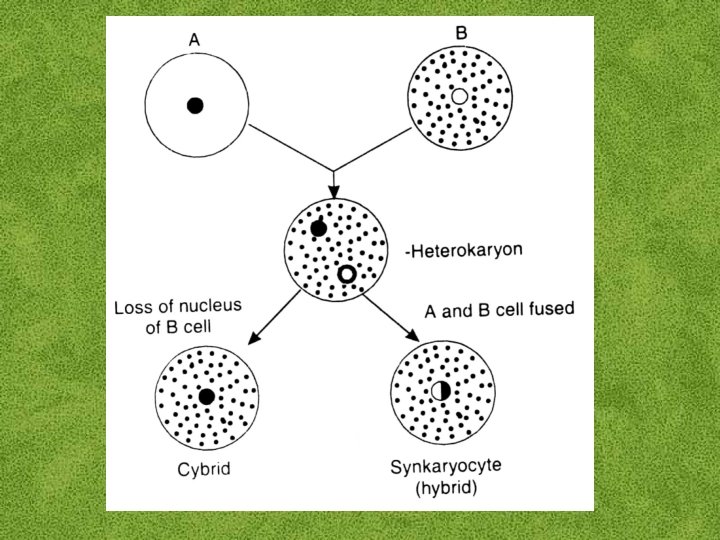
FUSION PRODUCTS Cells containing nonidentical nuclei are refered to as “heterokaryons” or “heterokaryocytes”. The fusion of nuclei in a binucleate heterokaryon results in the formation of a true hybrid protoplast or “synkaryocyte”.

The fusion of two protoplasts from the same culture results in a “homokaryon”. Frequently genetic information is lost from one of the nuclei. If one nucleus completely disappears, the cytoplasm of the two parental protoplasts are still hybridized, and the fusion product is known as a “cybrid” (cytoplasmic hybrid) or “ heteroplast”.



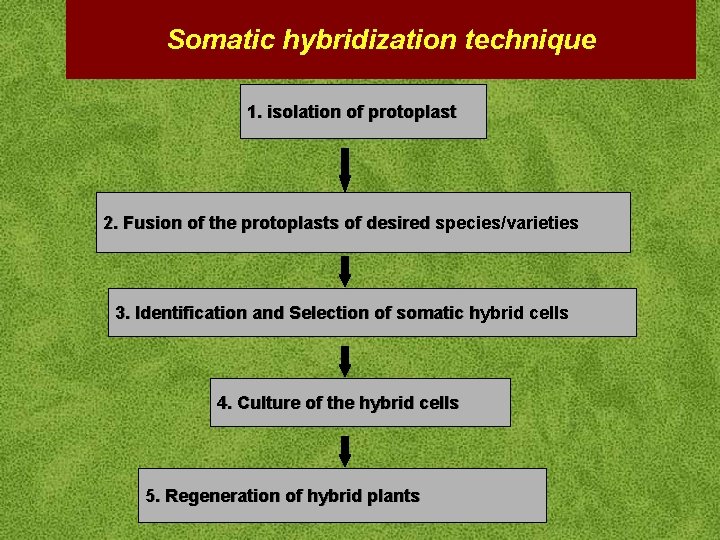
Somatic hybridization technique 1. isolation of protoplast 2. Fusion of the protoplasts of desired species/varieties 3. Identification and Selection of somatic hybrid cells 4. Culture of the hybrid cells 5. Regeneration of hybrid plants

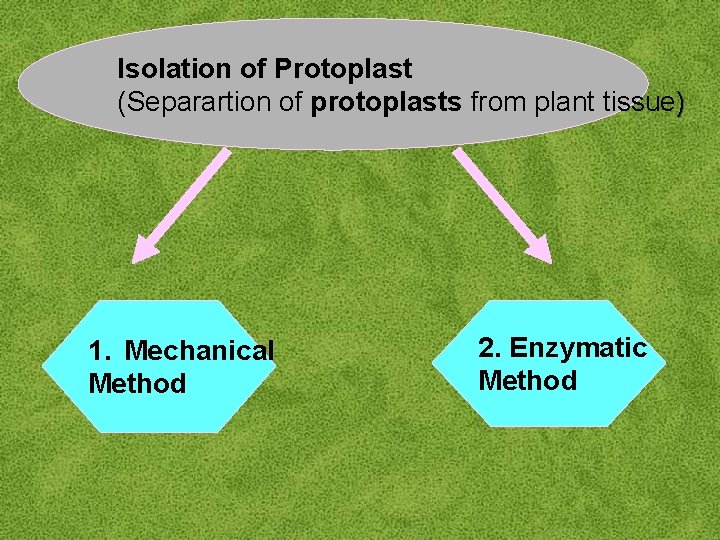
Isolation of Protoplast (Separartion of protoplasts from plant tissue) 1. Mechanical Method 2. Enzymatic Method

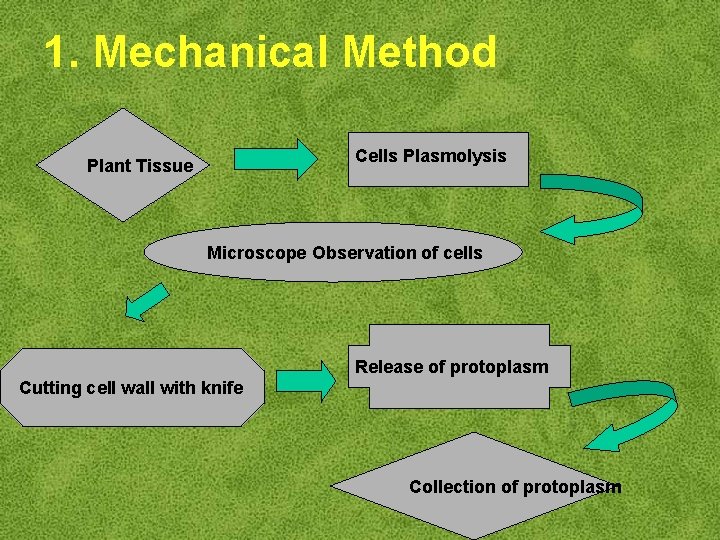
1. Mechanical Method Cells Plasmolysis Plant Tissue Microscope Observation of cells Release of protoplasm Cutting cell wall with knife Collection of protoplasm

1. Mechanical Method Used for vacuolated cells like onion bulb scale, radish and beet root tissues Low yield of protoplast Laborious and tedious process Low protoplast viability

Enzymatic Method Leaf sterlization, removal of epidermis Plasmolysed cells Pectinase +cellulase Protoplasm released Isolated Protoplasm Release of isolated cells cellulase Protoplasm released

Enzymatic Method Used for variety of tissues and organs including leaves, petioles, fruits, roots, coleoptiles, hypocotyls, stem, shoot apices, embryo microspores Mesophyll tissue - most suitable source High yield of protoplast Easy to perform More protoplast viability

Protoplast Fusion (Fusion of protoplasts of two different genomes) 1. Spontaneous Fusion Intraspecific Intergeneric 2. Induced Fusion Chemofusion Mechanical Fusion Electrofusion

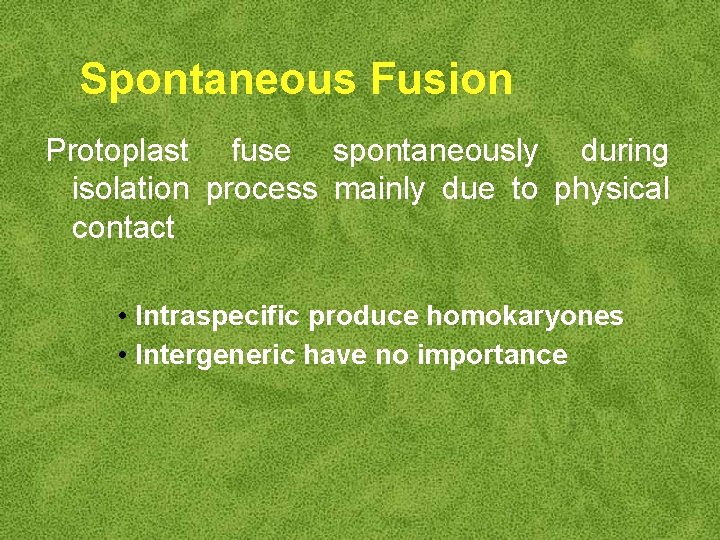
Spontaneous Fusion Protoplast fuse spontaneously during isolation process mainly due to physical contact • Intraspecific produce homokaryones • Intergeneric have no importance

Induced Fusion Chemofusion- fusion induced by chemicals • Types of fusogens • PEG • Na. No 3 • Ca 2+ ions • Polyvinyl alcohol

Induced Fusion Mechanical Fusion- Physical fusion of protoplasts under microscope by using micromanipulator and perfusion micropipette. Electrofusion- Fusion induced by electrical stimulation • Pearl chain of protoplasts is formed by low strength electric field (10 kv m-1) • Fusion of protoplasts of pearl chain is induced by the application of high strength electric field (100 kv m-1) for few microseconds.

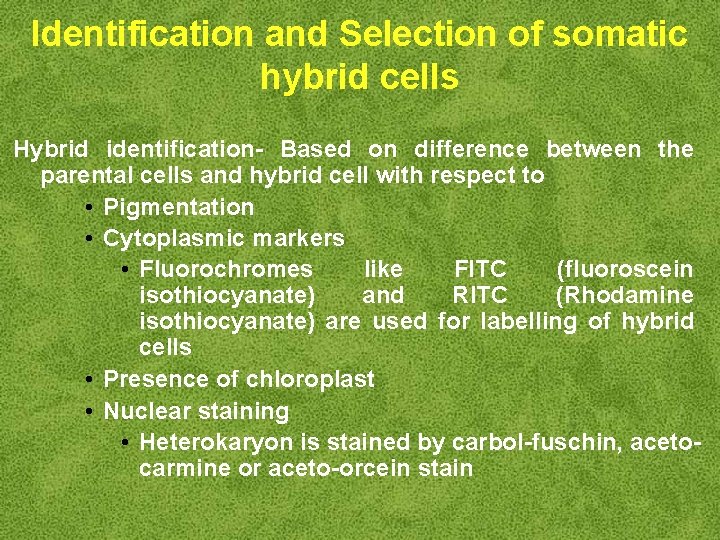
Identification and Selection of somatic hybrid cells Hybrid identification- Based on difference between the parental cells and hybrid cell with respect to • Pigmentation • Cytoplasmic markers • Fluorochromes like FITC (fluoroscein isothiocyanate) and RITC (Rhodamine isothiocyanate) are used for labelling of hybrid cells • Presence of chloroplast • Nuclear staining • Heterokaryon is stained by carbol-fuschin, acetocarmine or aceto-orcein stain

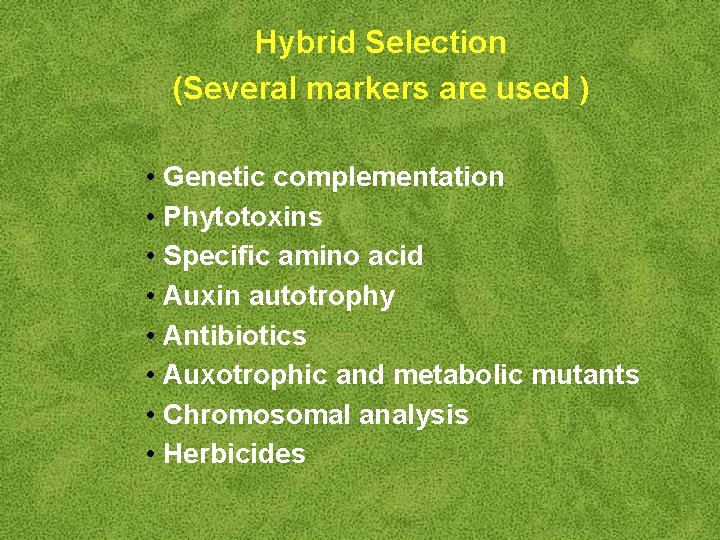
Hybrid Selection (Several markers are used ) • Genetic complementation • Phytotoxins • Specific amino acid • Auxin autotrophy • Antibiotics • Auxotrophic and metabolic mutants • Chromosomal analysis • Herbicides

Culture of the hybrid cells Hybrid cells are cultured on suitable medium provided with the appropriate culture conditions.

Regeneration of hybrid plants Plants are induced to regenerate from hybrid calli These hybrid plants must be at least partially fertile, in addition to having some useful property, to be of any use in breeding schemes.

Advantages of somatic hybridization Production of novel interspecific and intergenic hybrid Pomato (Hybrid of potato and tomato) Production of fertile diploids and polypoids from sexually sterile haploids, triploids and aneuploids Transfer gene for disease resistance, abiotic stress resistance, herbicide resistance and many other quality characters



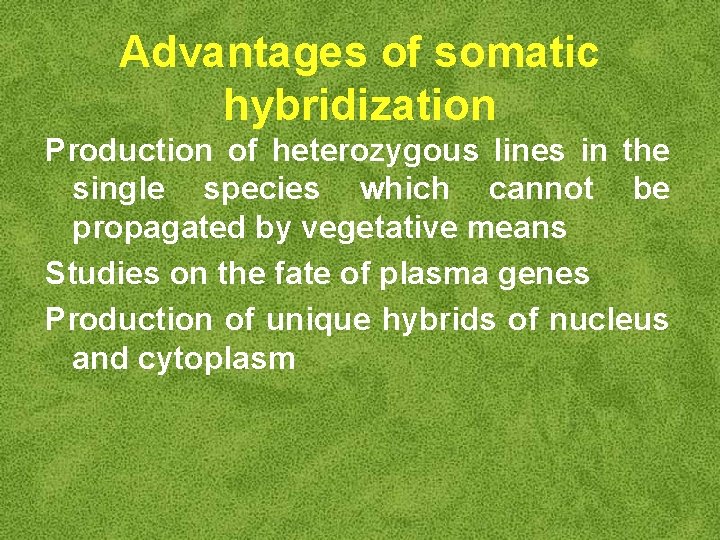
Advantages of somatic hybridization Production of heterozygous lines in the single species which cannot be propagated by vegetative means Studies on the fate of plasma genes Production of unique hybrids of nucleus and cytoplasm

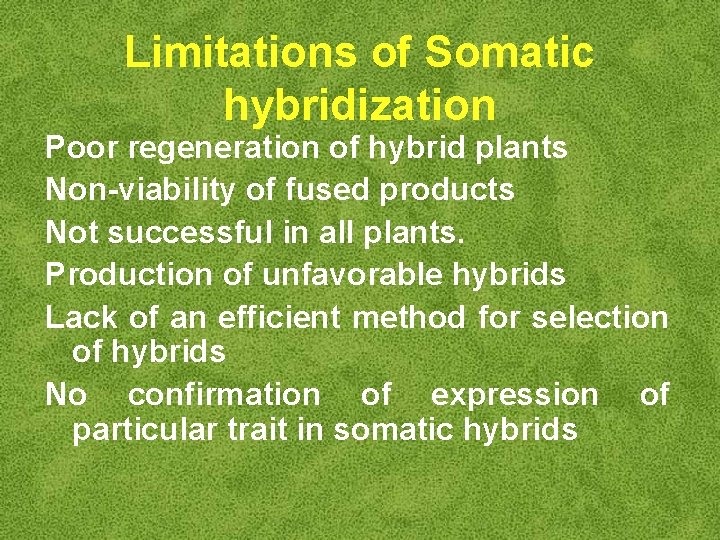
Limitations of Somatic hybridization Poor regeneration of hybrid plants Non-viability of fused products Not successful in all plants. Production of unfavorable hybrids Lack of an efficient method for selection of hybrids No confirmation of expression of particular trait in somatic hybrids
 Dog clip in suspension therapy
Dog clip in suspension therapy Suspension culture definition
Suspension culture definition Cell city analogy project
Cell city analogy project Advantages of diaphragm cell
Advantages of diaphragm cell Site:slidetodoc.com
Site:slidetodoc.com Prokaryotic
Prokaryotic Plant vs animal mitosis
Plant vs animal mitosis Lithium ion battery reaction equation
Lithium ion battery reaction equation Dry cell vs wet cell
Dry cell vs wet cell What is an organelle
What is an organelle What is the function of cell wall in plant cell
What is the function of cell wall in plant cell Plant cell and animal cell diagram
Plant cell and animal cell diagram Animal vs plant cell
Animal vs plant cell Cell wall cell membrane
Cell wall cell membrane Morphology of cells in culture
Morphology of cells in culture Cell line vs cell strain
Cell line vs cell strain Cell city project animal cell
Cell city project animal cell Primary voltaic cell
Primary voltaic cell Difference between bacteria and plant cell
Difference between bacteria and plant cell Cell-cell junction
Cell-cell junction Cell-cell junction
Cell-cell junction What cell organelle is like lysol spray cleaning the cell
What cell organelle is like lysol spray cleaning the cell Cell cycle and cell division
Cell cycle and cell division Life
Life Carbohydrate side chain
Carbohydrate side chain Chapter 4 cell theory and cell study
Chapter 4 cell theory and cell study Parts of a cell graphic organizer
Parts of a cell graphic organizer Idealized animal cell and plant cell
Idealized animal cell and plant cell Walker cell and hadley cell
Walker cell and hadley cell Prokaryote vs eukaryote worksheet
Prokaryote vs eukaryote worksheet Cell cycle and cell division
Cell cycle and cell division Biology.arizona.edu/cell bio/activities/cell cycle/01.html
Biology.arizona.edu/cell bio/activities/cell cycle/01.html Cell cycle and cell division
Cell cycle and cell division Matlab variable in string
Matlab variable in string Electrolysis vs voltaic cell
Electrolysis vs voltaic cell Flexible covering of an animal cell
Flexible covering of an animal cell Bioreactor considerations for animal cell culture
Bioreactor considerations for animal cell culture Hira abbas
Hira abbas Primary cell culture
Primary cell culture Procedure for isolation of cells for in vitro culture
Procedure for isolation of cells for in vitro culture Cell culture
Cell culture Cell culture
Cell culture Stem cell culture
Stem cell culture Sterile technique cell culture
Sterile technique cell culture Culture includes
Culture includes Individual culture traits combine to form culture patterns.
Individual culture traits combine to form culture patterns. Batch culture vs continuous culture
Batch culture vs continuous culture Fed-batch
Fed-batch Individualistic culture definition
Individualistic culture definition Indian culture vs american culture
Indian culture vs american culture Stroke culture method
Stroke culture method Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram A sub-culture group
A sub-culture group Popular culture examples
Popular culture examples Stab culture and stroke culture
Stab culture and stroke culture Folk culture and popular culture venn diagram
Folk culture and popular culture venn diagram Inert organizational culture
Inert organizational culture Anaerobic culture method
Anaerobic culture method Describe lawn culture and surface plating
Describe lawn culture and surface plating Laying the foundation for a quality culture
Laying the foundation for a quality culture Surface culture deep culture and esol
Surface culture deep culture and esol Suspension example
Suspension example Suspension vs solution
Suspension vs solution Suspension vs solution
Suspension vs solution Termodinamicamente inestable
Termodinamicamente inestable Disadvantage of suspension
Disadvantage of suspension Unibody suspension
Unibody suspension Table de roue de suspension
Table de roue de suspension Are aphids suspension feeders
Are aphids suspension feeders Scattering of light in suspension
Scattering of light in suspension Suspension solucion y coloide
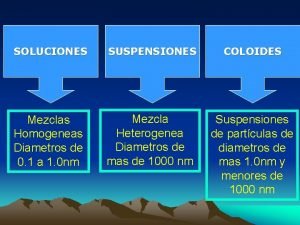
Suspension solucion y coloide Damping coefficient c
Damping coefficient c Cuadro comparativo de suspensiones coloides y soluciones
Cuadro comparativo de suspensiones coloides y soluciones Dialisis en quimica
Dialisis en quimica Hotchkiss rear suspension
Hotchkiss rear suspension General characteristics of dispersed system
General characteristics of dispersed system Difference between deflocculated and flocculated suspension
Difference between deflocculated and flocculated suspension Suspension mixture
Suspension mixture El agua mineral es disoluciones coloides o suspensiones
El agua mineral es disoluciones coloides o suspensiones Sssc suspension list
Sssc suspension list Electrodynamic suspension
Electrodynamic suspension Examples of benthic organisms
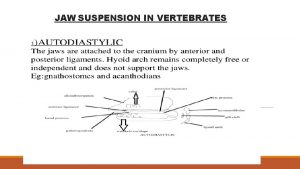
Examples of benthic organisms Streptostylic jaw suspension
Streptostylic jaw suspension Sedentary suspension feeders
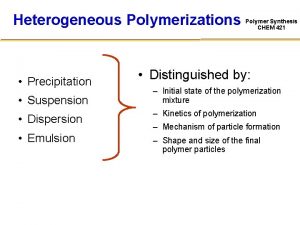
Sedentary suspension feeders Suspension emulsion dispersion
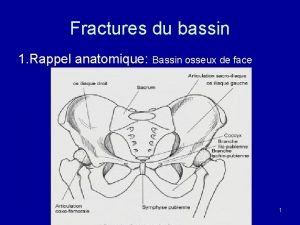
Suspension emulsion dispersion Classification de tile bassin
Classification de tile bassin Suspension en hamac fracture du bassin
Suspension en hamac fracture du bassin Dry suspension examples
Dry suspension examples Suspension de moto cinématique corrigé
Suspension de moto cinématique corrigé Suspension solucion y coloide
Suspension solucion y coloide Suspension solucion y coloide
Suspension solucion y coloide Colloidal state
Colloidal state Suspension cloning
Suspension cloning Roll center solid axle
Roll center solid axle Electronic suspension system
Electronic suspension system Chapter 75 suspension system technology
Chapter 75 suspension system technology Suspension mixture
Suspension mixture All acids owe their chemical reactivity to
All acids owe their chemical reactivity to Automotive steering, suspension and alignment
Automotive steering, suspension and alignment Automatic air suspension
Automatic air suspension Bose magnetic suspension
Bose magnetic suspension Heterogeneous aqueous systems
Heterogeneous aqueous systems Suspension feeding
Suspension feeding Suspension trauma treatment
Suspension trauma treatment Imágenes de mezclas homogéneas
Imágenes de mezclas homogéneas Floculacion controlada
Floculacion controlada Suspension bridge out of popsicle sticks
Suspension bridge out of popsicle sticks Wurster air suspension apparatus
Wurster air suspension apparatus Method of microencapsulation slideshare
Method of microencapsulation slideshare Examples of suspension
Examples of suspension Triceps cuff suspension
Triceps cuff suspension Cheliceriforms
Cheliceriforms Examples of extemporaneous compounding
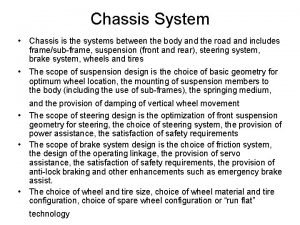
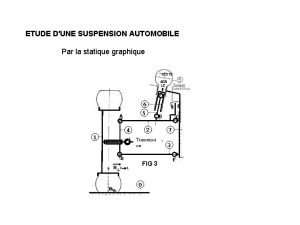
Examples of extemporaneous compounding étude suspension automobile
étude suspension automobile Hydrophillic colloids
Hydrophillic colloids Suspension definition pharmacy
Suspension definition pharmacy Coronal approach
Coronal approach Indiffusible solids
Indiffusible solids Trisulfapyrimidine oral suspension
Trisulfapyrimidine oral suspension Magnetic levitation inverter chiller supplier
Magnetic levitation inverter chiller supplier Define suspension of disbelief
Define suspension of disbelief Secondary retention and resistance form
Secondary retention and resistance form Lausd suspension matrix
Lausd suspension matrix Kissing nodules
Kissing nodules Niebla es un coloide
Niebla es un coloide Closed reduction traction
Closed reduction traction Suspension y extincion del contrato de trabajo
Suspension y extincion del contrato de trabajo Diffusible and indiffusible solids
Diffusible and indiffusible solids Suspencion incompleta
Suspencion incompleta Introduction sur la suspension du contrat de travail
Introduction sur la suspension du contrat de travail Suspension grouting
Suspension grouting Canesten thrush cream boots
Canesten thrush cream boots Solutions, suspensions and colloids activity
Solutions, suspensions and colloids activity Is carbon dioxide in lemonade a suspension
Is carbon dioxide in lemonade a suspension Classification of suspension
Classification of suspension