CELL STRUCTURE AND FUNCTIONS BIOMOLECULES CELL STRUCTURE AND
CELL: STRUCTURE AND FUNCTIONS BIOMOLECULES
CELL: STRUCTURE AND FUNCTIONS
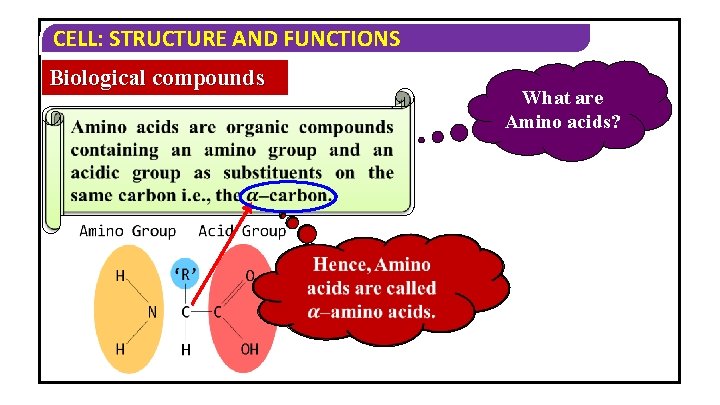
CELL: STRUCTURE AND FUNCTIONS Biological compounds What are Amino acids?
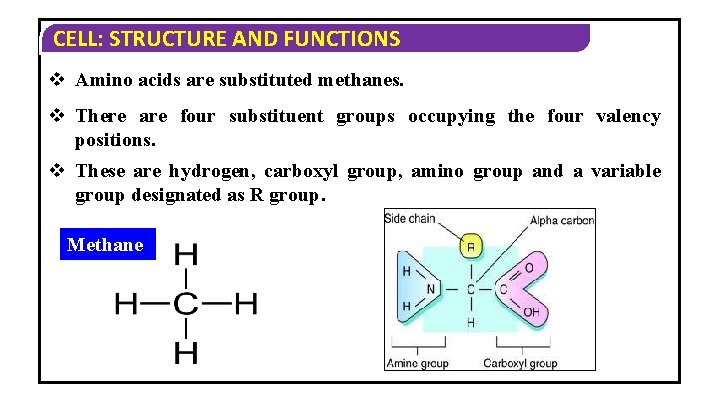
CELL: STRUCTURE AND FUNCTIONS v Amino acids are substituted methanes. v There are four substituent groups occupying the four valency positions. v These are hydrogen, carboxyl group, amino group and a variable group designated as R group. Methane
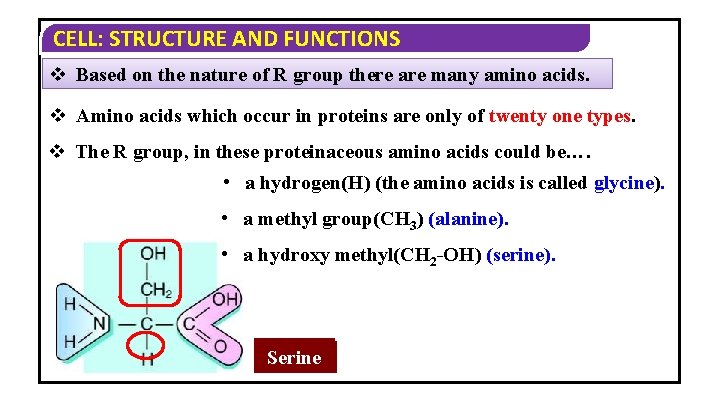
CELL: STRUCTURE AND FUNCTIONS v Based on the nature of R group there are many amino acids. v Amino acids which occur in proteins are only of twenty one types. v The R group, in these proteinaceous amino acids could be…. • a hydrogen(H) (the amino acids is called glycine). • a methyl group(CH 3) (alanine). • a hydroxy methyl(CH 2 -OH) (serine). Glycine Alanine Serine
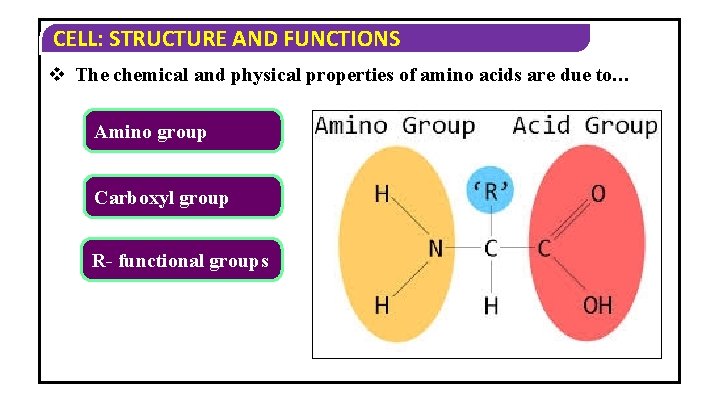
CELL: STRUCTURE AND FUNCTIONS v The chemical and physical properties of amino acids are due to… Amino group Carboxyl group R- functional groups
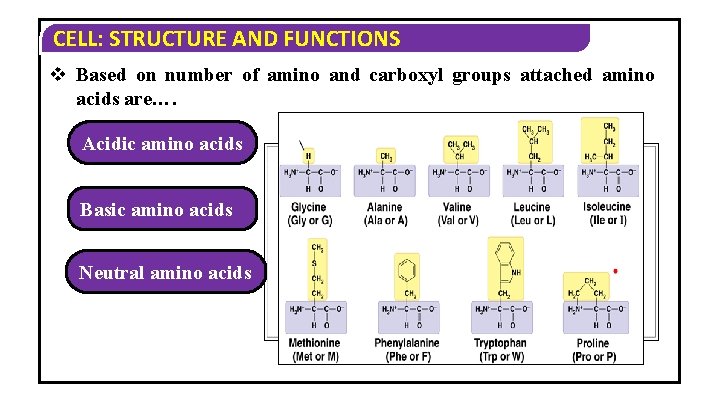
CELL: STRUCTURE AND FUNCTIONS v Based on number of amino and carboxyl groups attached amino acids are…. Acidic amino acids Basic amino acids Neutral amino acids
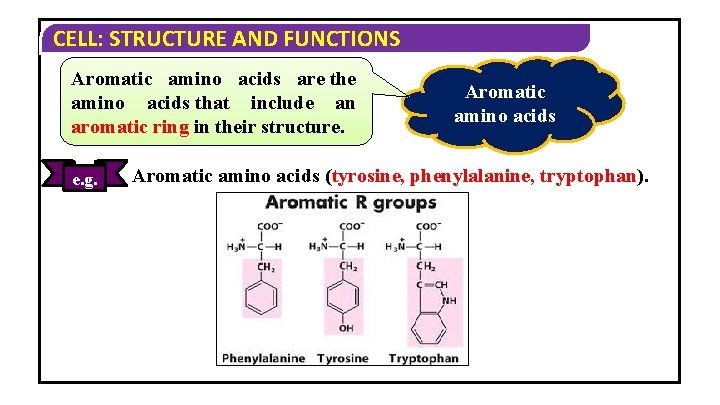
CELL: STRUCTURE AND FUNCTIONS Aromatic amino acids are the amino acids that include an aromatic ring in their structure. e. g. Aromatic amino acids (tyrosine, phenylalanine, tryptophan).
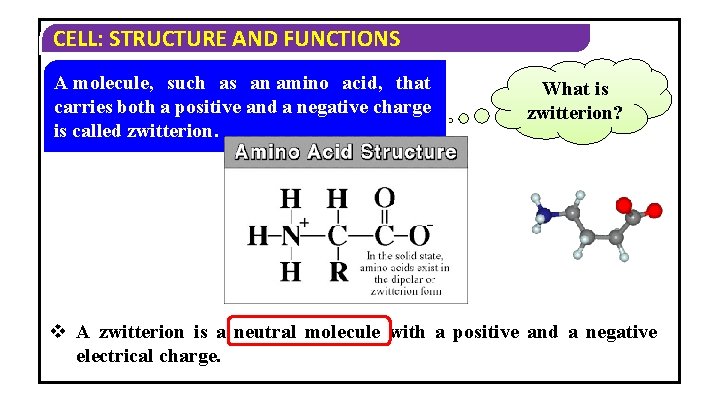
CELL: STRUCTURE AND FUNCTIONS A molecule, such as an amino acid, that carries both a positive and a negative charge is called zwitterion. What is zwitterion? v A zwitterion is a neutral molecule with a positive and a negative electrical charge.
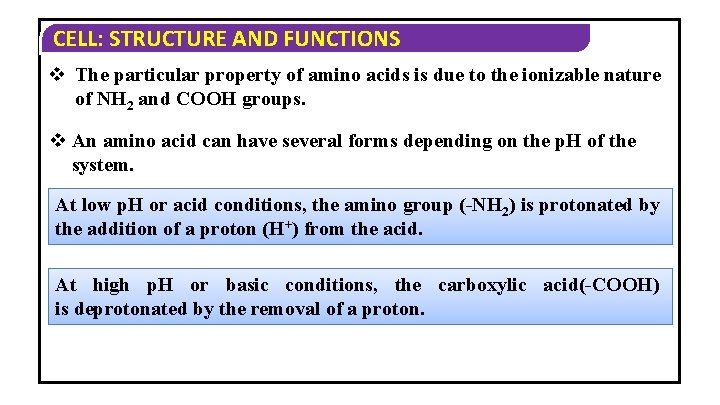
CELL: STRUCTURE AND FUNCTIONS v The particular property of amino acids is due to the ionizable nature of NH 2 and COOH groups. v An amino acid can have several forms depending on the p. H of the system. At low p. H or acid conditions, the amino group (-NH 2) is protonated by the addition of a proton (H+) from the acid. At high p. H or basic conditions, the carboxylic acid(-COOH) is deprotonated by the removal of a proton.
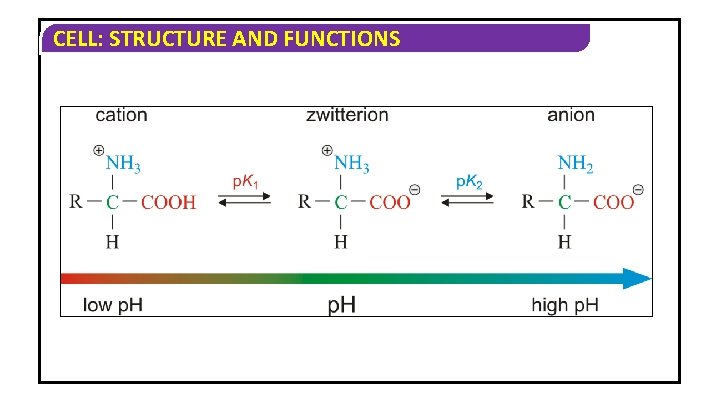
CELL: STRUCTURE AND FUNCTIONS
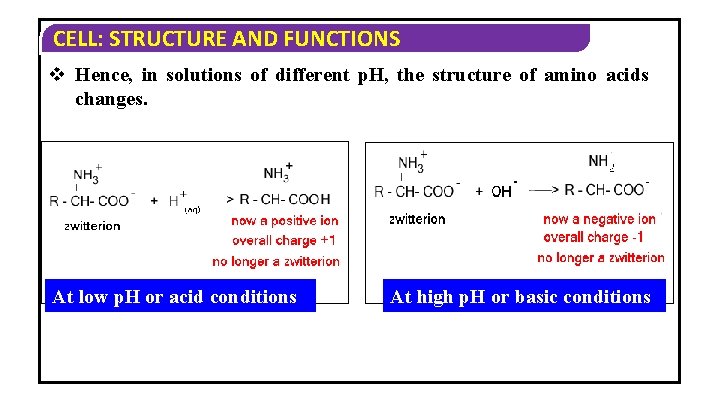
CELL: STRUCTURE AND FUNCTIONS v Hence, in solutions of different p. H, the structure of amino acids changes. At low p. H or acid conditions At high p. H or basic conditions
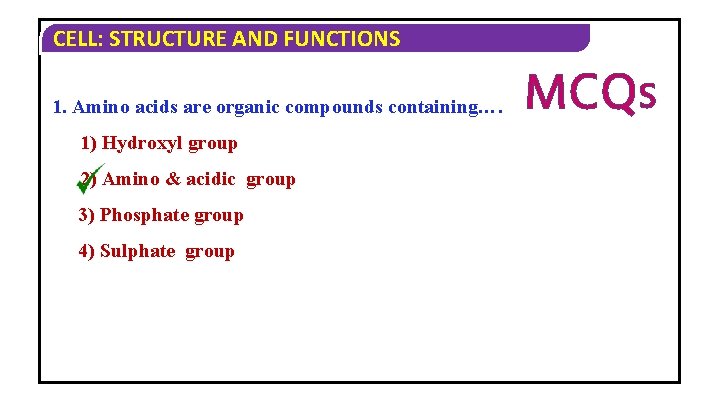
CELL: STRUCTURE AND FUNCTIONS 1. Amino acids are organic compounds containing…. 1) Hydroxyl group 2) Amino & acidic group 3) Phosphate group 4) Sulphate group MCQS
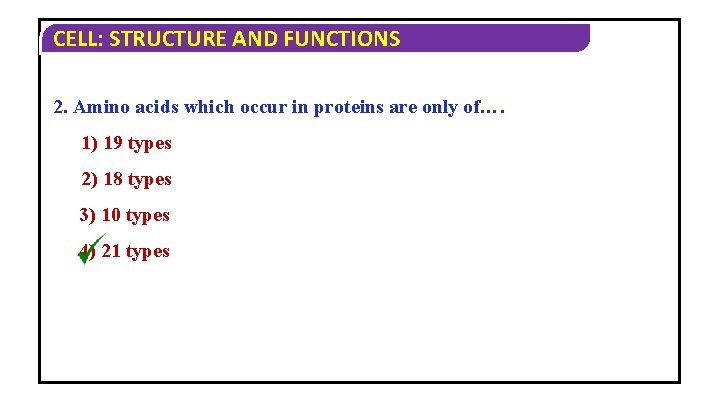
CELL: STRUCTURE AND FUNCTIONS 2. Amino acids which occur in proteins are only of…. 1) 19 types 2) 18 types 3) 10 types 4) 21 types
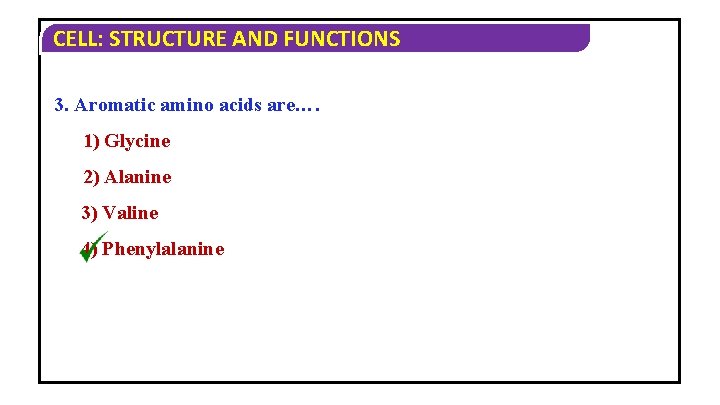
CELL: STRUCTURE AND FUNCTIONS 3. Aromatic amino acids are…. 1) Glycine 2) Alanine 3) Valine 4) Phenylalanine
CELL: STRUCTURE AND FUNCTIONS
CELL: STRUCTURE AND FUNCTIONS Lipids What are Lipids? Lipids are class of organic compounds that are fatty acids or their derivatives. Ø They are insoluble in water but soluble in organic solvents.

CELL: STRUCTURE AND FUNCTIONS e. g. Natural oils, Waxes, and Steroids.
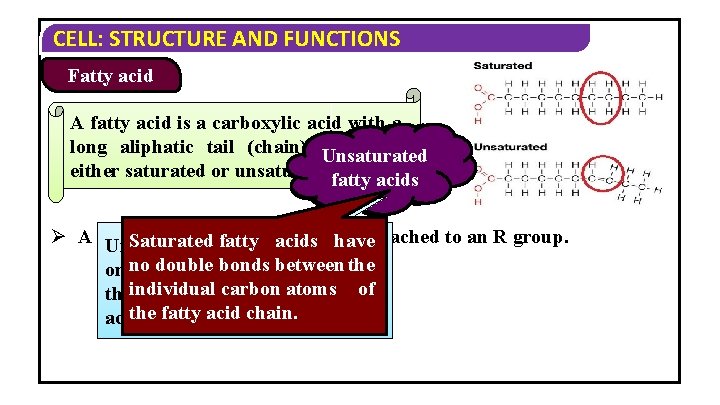
CELL: STRUCTURE AND FUNCTIONS Fatty acid A fatty acid is a carboxylic acid with a long aliphatic tail (chain), Unsaturated which is Saturated either saturated or unsaturated. fattyacids fatty Ø A fatty acid has afatty carboxyl group attached to an R group. Saturated fatty acids have Unsaturated acids have one double bonds between the or no more double bonds between carbonofatoms of theindividual carbon atoms the fatty acid chain.
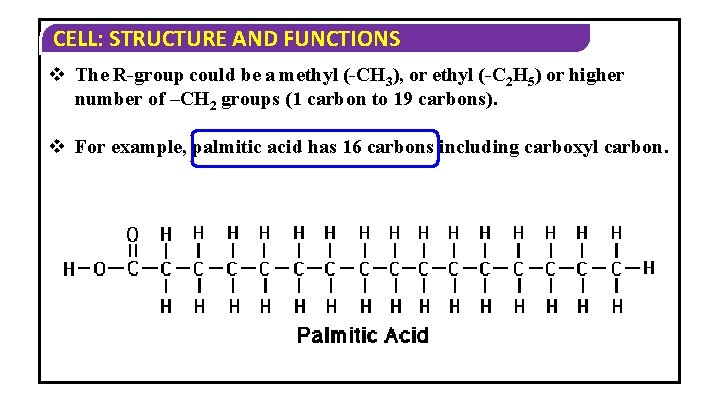
CELL: STRUCTURE AND FUNCTIONS v The R-group could be a methyl (-CH 3), or ethyl (-C 2 H 5) or higher number of –CH 2 groups (1 carbon to 19 carbons). v For example, palmitic acid has 16 carbons including carboxyl carbon.
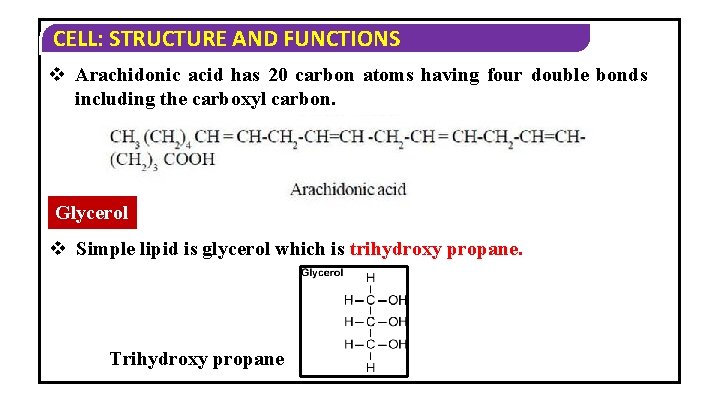
CELL: STRUCTURE AND FUNCTIONS v Arachidonic acid has 20 carbon atoms having four double bonds including the carboxyl carbon. Glycerol v Simple lipid is glycerol which is trihydroxy propane. Trihydroxy propane
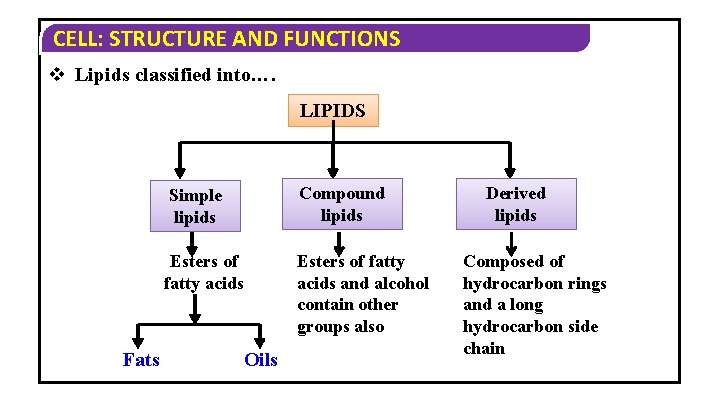
CELL: STRUCTURE AND FUNCTIONS v Lipids classified into…. LIPIDS Fats Simple lipids Compound lipids Esters of fatty acids and alcohol contain other groups also Oils Derived lipids Composed of hydrocarbon rings and a long hydrocarbon side chain
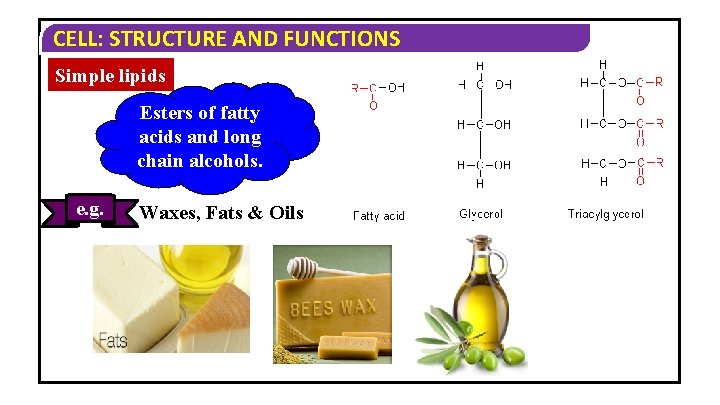
CELL: STRUCTURE AND FUNCTIONS Simple lipids Esters of fatty acids and long chain alcohols. e. g. Waxes, Fats & Oils
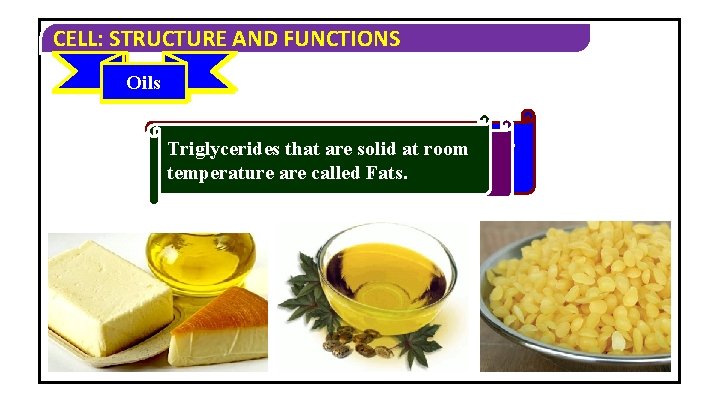
CELL: STRUCTURE AND FUNCTIONS Waxes Oils Fats A wax is a simple lipid which isroom an ester Triglycerides that are liquid solid atatroom Triglycerides that of a long-chain temperature arealcohol called and Fats. a fatty acid. temperature are called Oils.
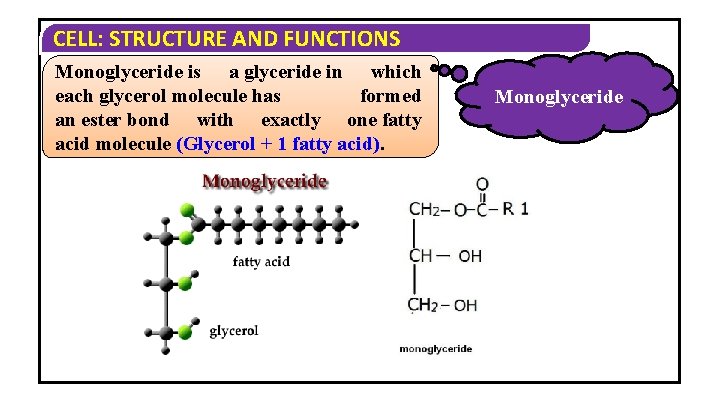
CELL: STRUCTURE AND FUNCTIONS Monoglyceride is a glyceride in which each glycerol molecule has formed an ester bond with exactly one fatty acid molecule (Glycerol + 1 fatty acid). Monoglyceride
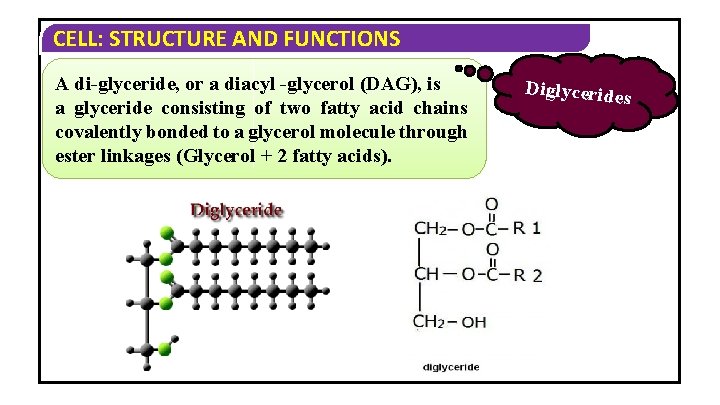
CELL: STRUCTURE AND FUNCTIONS A di-glyceride, or a diacyl -glycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages (Glycerol + 2 fatty acids). Diglycerides
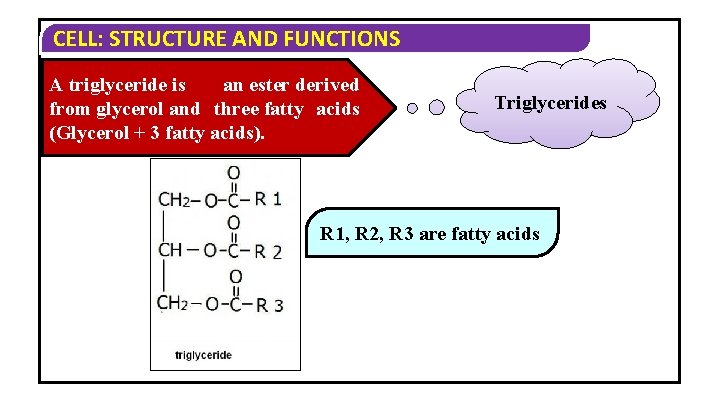
CELL: STRUCTURE AND FUNCTIONS A triglyceride is an ester derived from glycerol and three fatty acids (Glycerol + 3 fatty acids). Triglycerides R 1, R 2, R 3 are fatty acids
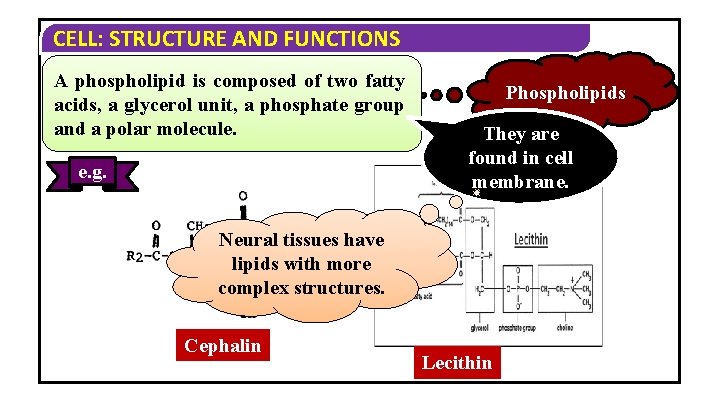
CELL: STRUCTURE AND FUNCTIONS A phospholipid is composed of two fatty acids, a glycerol unit, a phosphate group and a polar molecule. e. g. Phospholipids They are found in cell membrane. Neural tissues have lipids with more complex structures. Cephalin Lecithin
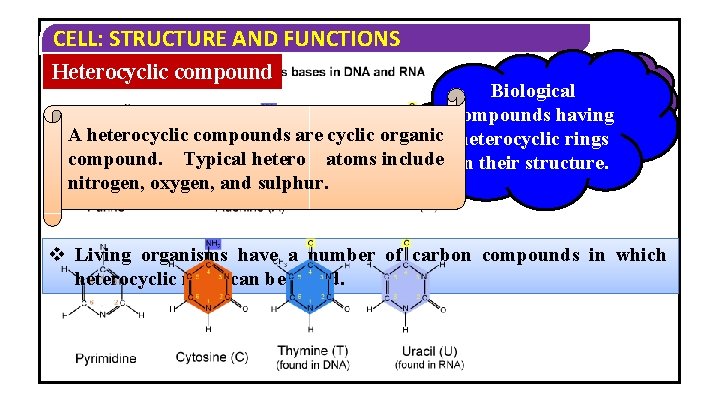
CELL: STRUCTURE AND FUNCTIONS Heterocyclic compound Heterocyclic Biological compounds having A heterocyclic compounds are cyclic organic heterocyclic rings compound. Typical hetero atoms include in their structure. nitrogen, oxygen, and sulphur. v Living organisms have a number of carbon compounds in which heterocyclic rings can be found.
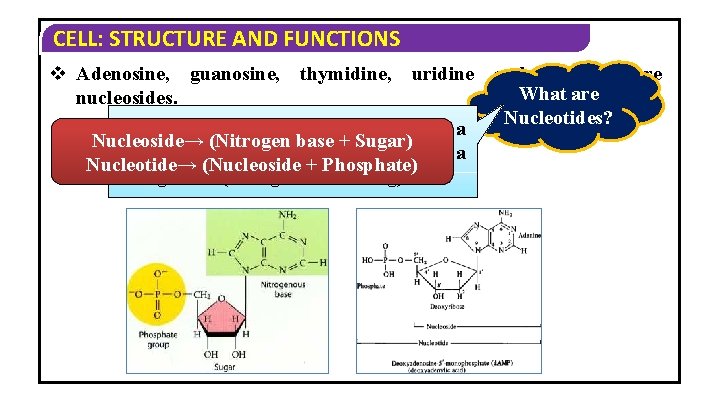
CELL: STRUCTURE AND FUNCTIONS v Adenosine, guanosine, thymidine, uridine and cytidine are What are nucleosides. Nucleotides? Nucleotides are molecules comprised of a Nucleoside→ (Nitrogen base + Sugar) sugar molecule, a phosphate unit and a Nucleotide→ (Nucleoside + Phosphate) nitrogenous (nitrogen-containing) base.
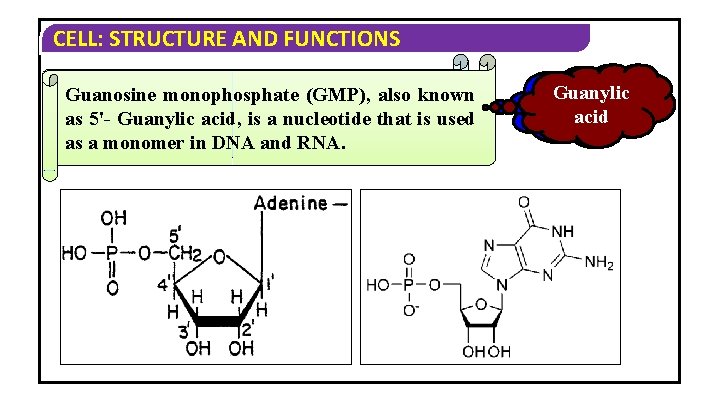
CELL: STRUCTURE AND FUNCTIONS Adenosine monophosphate(GMP), (AMP), Guanosine monophosphate also known as 5'-adenylic is a nucleotide as 5'- Guanylic acid, is a nucleotide that is used is as a monomer DNA and RNA. asused a monomer in DNAinand RNA. Adenylic Guanylic acid
CELL: STRUCTURE AND FUNCTIONS v Adenylic acid, thymidylic acid, guanylic acid, uridylic acid and cytidylic acid are nucleotides. v Nucleic acids like DNA and RNA consist of nucleotides only. v DNA and RNA function as genetic material.
CELL: STRUCTURE AND FUNCTIONS 1. Lipids are soluble in…. 1) Water 2) Organic solvents 3) Water & organic compounds 4) None of these MCQS
CELL: STRUCTURE AND FUNCTIONS 2. Triglycerides that are liquid at room temperature are…. 1) Fats 2) Oils 3) Waxes 4) Steroids
CELL: STRUCTURE AND FUNCTIONS 3. Glycerol is a …… 1) 1, 1, 1, trihydroxy propane 2) 1, 2, 2, trihydroxy propane 3) 1, 2, 3, trihydroxy propane 4) 1, 1, 2 trihydroxy propane
CELL: STRUCTURE AND FUNCTIONS 4. Number of carbon atoms in R group Arachidonic acid 1) 19 2) 20 3) 16 4) 15
CELL: STRUCTURE AND FUNCTIONS 5. A nucleoside is made up of…. 1) Sugar and Phosphate 2) Sugar and nitrogen base 3) Phosphate and nitrogen base 4) Sugar, phosphate and nitrogen base
CELL: STRUCTURE AND FUNCTIONS 6. Which of the following can functions as genetic material? 1) DNA 2) RNA 3) Both 1 & 2 4) Lipid

CELL: STRUCTURE AND FUNCTIONS Thank you…
- Slides: 39