Cell Signaling Lecture 12 NFk B NFk B

Cell Signaling Lecture 12
NFk. B �NFk. B (nuclear factor kappa beta) is a transcription factor that plays important roles in the immune system. � NFk. B regulates the expression of cytokines, inducible nitric oxide synthase (i. NOS)-the enzyme that produces nitric oxide which is toxic to bacterial cells. � inhibitors of apoptosis protein that prevent cell death and effector enzymes in response to ligation of many receptors involved in immunity including Tcell receptors (TCRs), B-cell receptors (BCRs) and members of the Toll-like receptor/IL-1 receptor super family. �NFk. B also plays a role in the development and the activity of a number of tissues including the central nervous system, liver cells etc. �Moreover, pathological dysregulation of NFk. B is
NFk. B family � In mammals, the NFk. B family is composed of five related transcription factors: p 50, p 52, Rel. A (p 65), c-Rel and Rel. B. � These transcription factors are related through an Nterminal, 300 amino acid, DNA binding/dimerization domain, called the Rel homology domain (RHD), through which they can form homodimers and heterodimers that bind to 9 -10 base pair DNA sites, known as k. B sites, in the promoters and enhancer regions of genes, thereby modulating gene expression. � Rel. A, c-Rel and Rel. B contain C-terminal transcriptional activation domains (TADs), which enable them to activate target gene expression. In contrast, p 50 and p 52 do not contain C-terminal TADs; therefore, p 50 and p 52 homodimers repress transcription unless they are bound to a protein containing a TAD, such as Rel. A, c-Rel or Rel. B or Bcl-3 (a related transcriptional coactivator). .

�NFk. B’s transcriptional activity is silenced by interactions with inhibitory Ik. B proteins present in the cytoplasm. �There are currently seven identified Ik. B family members - Ik. Ba, Ik. Bb, Bcl 3, Ik. Be, Ik. Bg and the precursor proteins p 100 and p 105
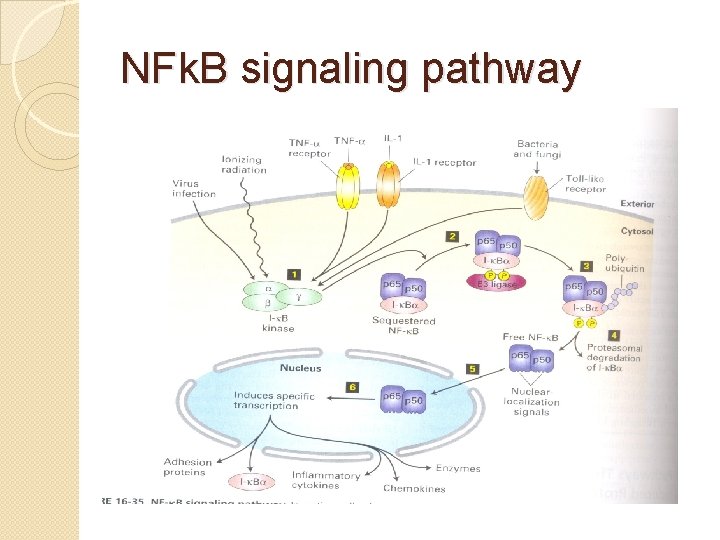
NFk. B signaling pathway
� There are two signaling pathways leading to the activation of NFk. B known as the canonical pathway (or classical) and the non-canonical pathway (or alternative pathway). � The common regulatory step in both of these cascades is activation of an Ik. B kinase (IKK) complex consisting of catalytic kinase subunits (IKKa and/or IKKb) and the regulatory non-enzymatic scaffold protein NEMO (NF-kappa B essential modulator also known as IKKg). � Activation of NFk. Bdimers is due to IKK-mediated phosphorylation-induced proteasomal degradation of the Ik. B inhibitor enabling the active NFk. Btranscription factor subunits to translocate to the nucleus and induce target gene expression. � NFk. B activation leads to the expression of the Ik. Ba gene, which consequently sequesters NFk. B subunits and terminates transcriptional activity unless a persistent activation signal is present.
Conical/classical �In the canonical signaling pathway, binding of ligand to a cell surface receptor such as a member of the Toll-like receptor superfamily leads to the recruitment of adaptors (such as TRAF) to the cytoplasmic domain of the receptor. �These adaptors in turn recruit the IKK complex which leads to phosphorylation and degradation of the Ik. B inhibitor. �The canonical pathway activates. NFk. B dimers comprising of Rel. A, c-Rel, Rel. B and p 50.
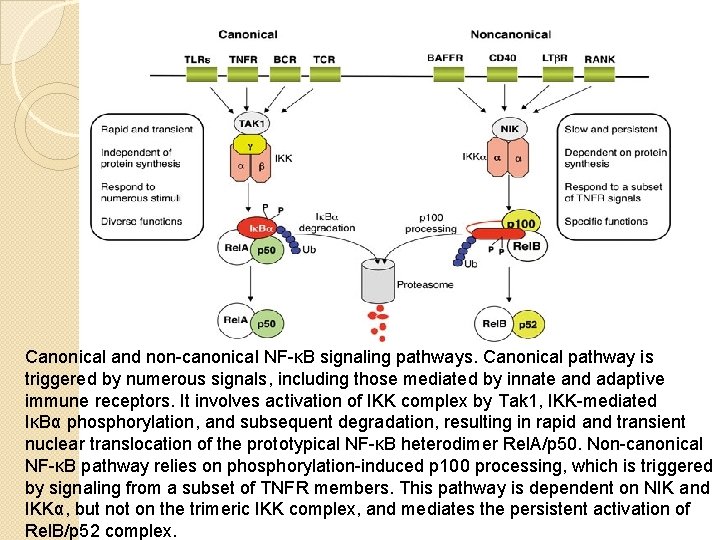
Canonical and non-canonical NF-κB signaling pathways. Canonical pathway is triggered by numerous signals, including those mediated by innate and adaptive immune receptors. It involves activation of IKK complex by Tak 1, IKK-mediated IκBα phosphorylation, and subsequent degradation, resulting in rapid and transient nuclear translocation of the prototypical NF-κB heterodimer Rel. A/p 50. Non-canonical NF-κB pathway relies on phosphorylation-induced p 100 processing, which is triggered by signaling from a subset of TNFR members. This pathway is dependent on NIK and IKKα, but not on the trimeric IKK complex, and mediates the persistent activation of Rel. B/p 52 complex.

Non-conical � The non-canonical pathway is responsible for the activation of p 100/Rel. B complexes and occurs during the development of lymphoid organs responsible for the generation of B and T lymphocytes. � Only a small number of stimuli are known to activate NFk. B via this pathway and these factors include lymphotoxin B and B cell activating factor (BAFF). � This pathway utilizes an IKK complex that comprises two IKKa subunits, but not NEMO. In the non-canonical pathway, ligand induced activation results in the activation of NFk. Binducing kinase (NIK), which phosphorylates and activates the IKKa complex, which in turn phosphorylates p 100 leading to the processing and liberation of the p 52/Rel. B active heterodimer. In contrast to p 100, p 105 undergoes constitutive cleavage to producep 50, whether p 105 can undergo inducible processing remains a contentious issue.
Role of NFk. B in diseases � � � Asthma is a chronic inflammatory disorder. The pathogenesis of asthma involves the persistent expression of pro-inflammatory cytokines, chemokines and other such inflammatory mediators. Many of these genes contain the k. B site for NFk. B within their promoters, suggesting that NFk. B plays a vital role in asthma. Indeed, increased NFk. B activity has been observed in the airways of asthmatic patients. NFk. B is also implicated in inflammatory bowel disease such as Crohn’s disease and ulcerative colitis. NFk. Bactivation is evident in biopsies from such patients and treatment of patients with steroids decreases NFk. B activity in biopsies as well as reducing the clinical symptoms of disease. Furthermore, NFk. B is involved in the pathophysiology of the autoimmune disorder rheumatoid arthritis (RA). NFk. B itself is upregulated in RA and cytokines such as TNFa that activate NFk. B are elevated in the synovial fluid of patients with RA. In addition to the roles that NFk. B plays in inflammatory diseases, constitutive activation of the NFk. B pathway is involved in some forms of cancer such as leukemia, lymphoma, colon cancer and ovarian cancer. Mutations that can lead to such tumors include those that inactivate Ik. B proteins as well as amplifications and rearrangements of genes encoding the NFk. Btranscription factor subunits. However, more commonly it is thought that changes in the upstream pathways that lead to NFk. Bactivation become deregulated in cancer.

Assignment topics �G 1: Herdgehog Signaling pathway in breast cancer development �G 2: RTK/Ras. MAP signaling pathway �G 3: Notch signaling pathway �G 4: PI 3 K/AKT pathway �G 5: EGFR and Ras Signaling pathway �G 6: Ras/Raf/MEK/ERK �G 7: NFk. B signaling pathway �G 8: JAK/STAT signaling pathway

Assignment should include � What is breast cancer? � Types of breast cancer � Stages of breast cancer � Epidemeology of this cancer world wide, South East Asia and Pakistan. Write in tabulated form � Introduce and explain your signaling pathway with schematic diagram or cycle. � What happens in breast cancer when this pathway gets disrupted and how? Include diagram or cycle � Potential treatments for making the pathway normal. � References with every information is required otherwise you will lose marks � Pages 10. � DEADline: 15 May

Table of statistics Country No. of Ethnicit Gender patients y /%age of populat ion affected Age (if given) Type of breast cancer Type of mutatio ns Referen ces For the American and European data, you should give the reference of a paper or data by US cancer research or UK cancer research For South East Asian and Pakistan data, include references from WHO, Shoukat Khannam or research papers.
- Slides: 13