Cell Respiration Mitochondria scanning electron microscope SEM by
Cell Respiration Mitochondria, scanning electron microscope (SEM) by Dr. David Furness

Life Requires Energy • Living cells require energy from outside sources • Organisms may get this energy from the sun, by consuming other organisms, or from heat and chemicals released by the earth.

• Energy enters most ecosystems from the sun. • Producers, such as green plants and algae, use that energy to generate polysaccharides like cellulose and starch from carbon dioxide.

• Consumers ingest and release energy locked in those molecules through the process of cell respiration. – A food web shows the flow of that energy.

• In biology, consumers are also referred to as heterotrophs. – From the Greek hetero- meaning “different”, and -troph meaning “nutrition. ” • Producers are referred to as autotrophs. – From the Greek auto- meaning “self”, and –troph meaning “nutrition”.
Potential and Kinetic Energy • Polysaccharides, proteins, and lipids all contain a great deal of stored, or potential energy. – This energy is not useful to the cell unless it can be converted to kinetic energy in a controlled manner and used to create some kind of cellular motion.
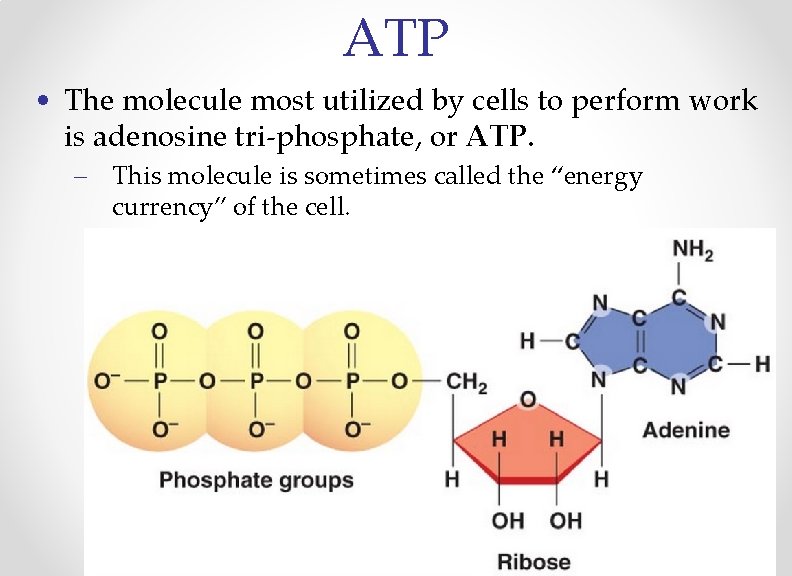
ATP • The molecule most utilized by cells to perform work is adenosine tri-phosphate, or ATP. – This molecule is sometimes called the “energy currency” of the cell.
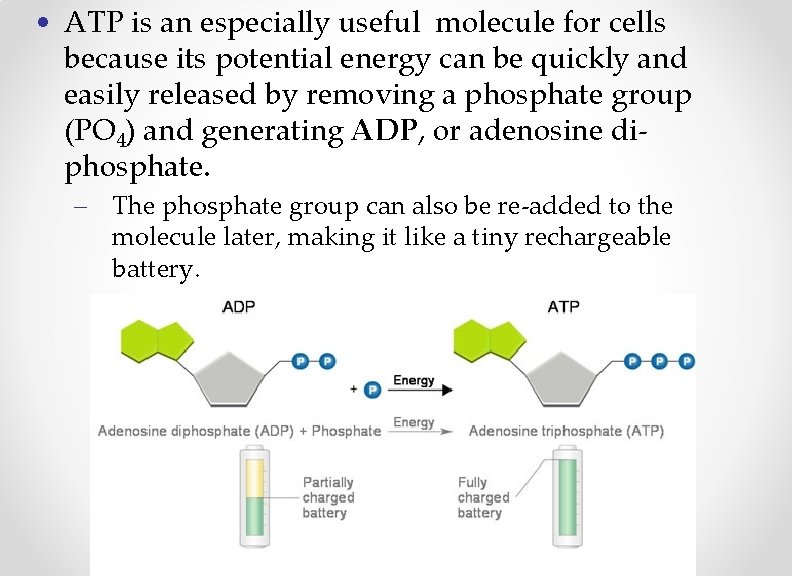
• ATP is an especially useful molecule for cells because its potential energy can be quickly and easily released by removing a phosphate group (PO 4) and generating ADP, or adenosine diphosphate. – The phosphate group can also be re-added to the molecule later, making it like a tiny rechargeable battery.
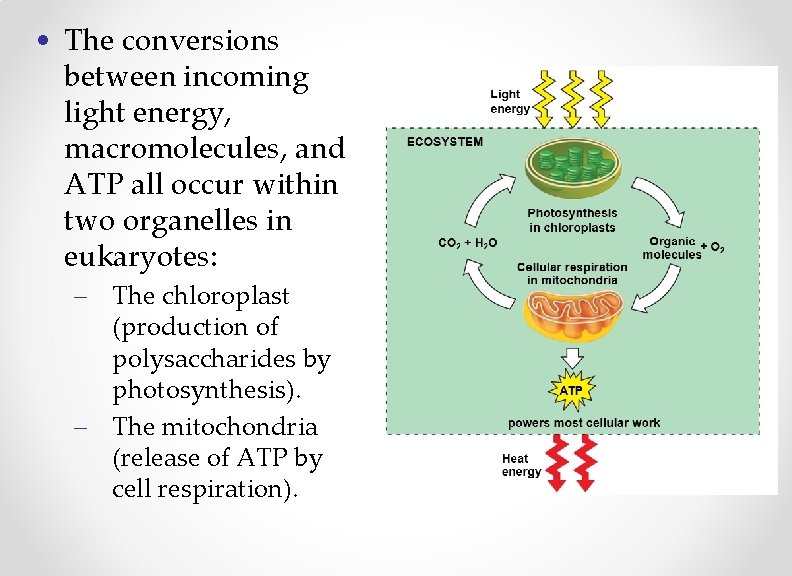
• The conversions between incoming light energy, macromolecules, and ATP all occur within two organelles in eukaryotes: – The chloroplast (production of polysaccharides by photosynthesis). – The mitochondria (release of ATP by cell respiration).
Glycolysis • The first part of cell respiration, called glycolysis, occurs within the cytosol outside of the mitochondria. – Glycolysis translates to “splitting of sugar. ”
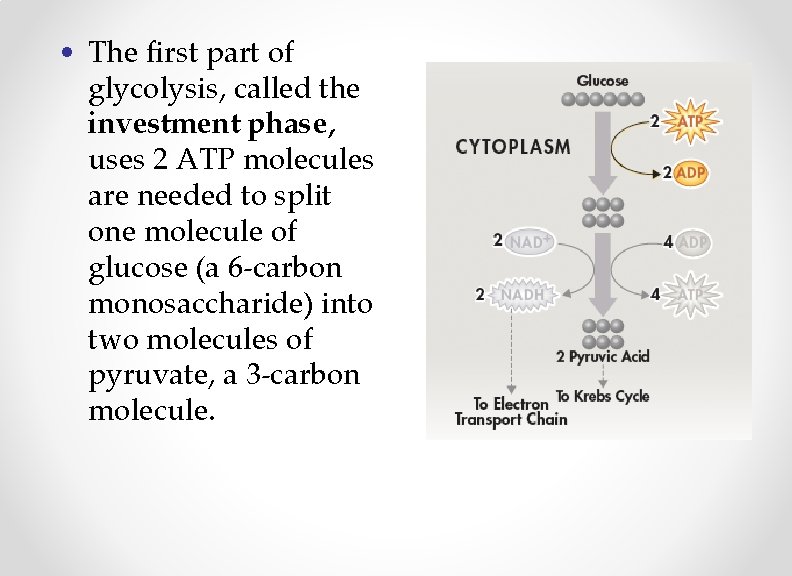
• The first part of glycolysis, called the investment phase, uses 2 ATP molecules are needed to split one molecule of glucose (a 6 -carbon monosaccharide) into two molecules of pyruvate, a 3 -carbon molecule.
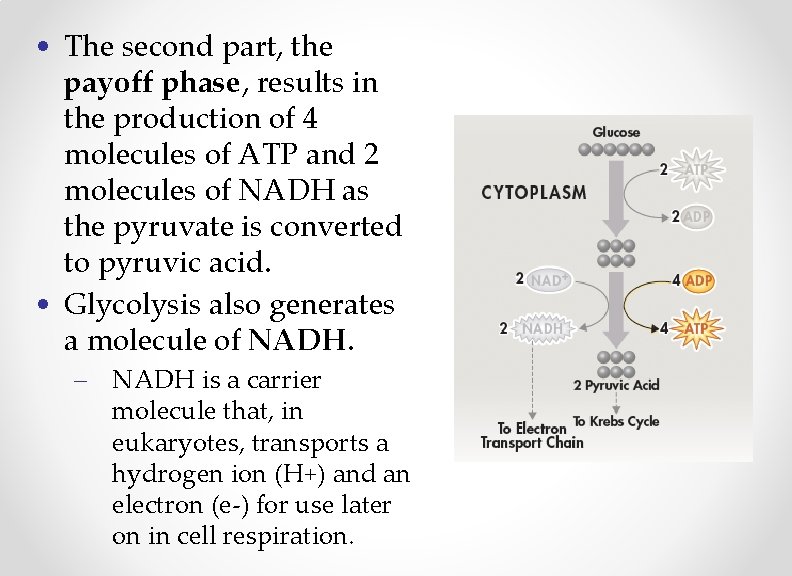
• The second part, the payoff phase, results in the production of 4 molecules of ATP and 2 molecules of NADH as the pyruvate is converted to pyruvic acid. • Glycolysis also generates a molecule of NADH. – NADH is a carrier molecule that, in eukaryotes, transports a hydrogen ion (H+) and an electron (e-) for use later on in cell respiration.
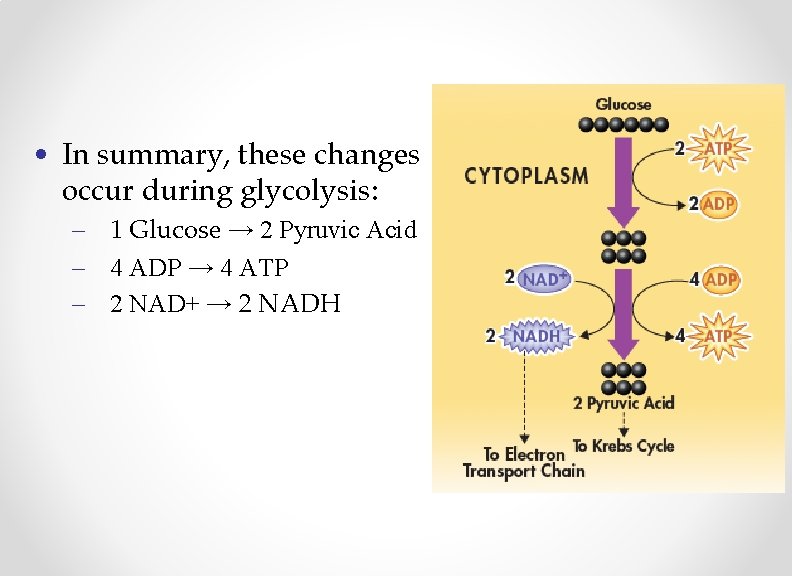
• In summary, these changes occur during glycolysis: – 1 Glucose → 2 Pyruvic Acid – 4 ADP → 4 ATP – 2 NAD+ → 2 NADH
• Glycolysis produces ATP very quickly, which is an advantage when the energy demands of the cell suddenly increase. • Glycolysis is anaerobic, meaning it does not require oxygen. – Some prokaryotes rely heavily on glycolysis, as they lack mitochondria needed to perform the rest of cell respiration. – Eukaryotes will also use glycolysis in situations where oxygen levels are insufficient. • The process of generating ATP primarily from glycolysis is called anaerobic fermentation.
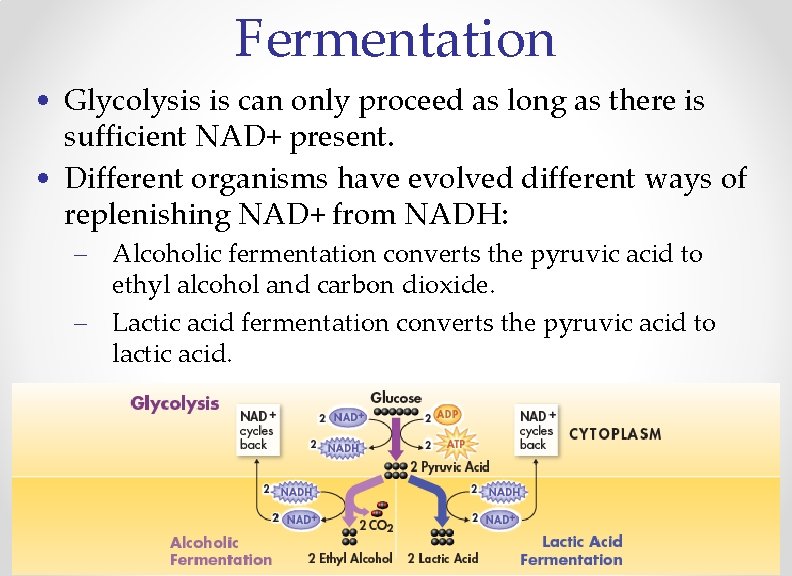
Fermentation • Glycolysis is can only proceed as long as there is sufficient NAD+ present. • Different organisms have evolved different ways of replenishing NAD+ from NADH: – Alcoholic fermentation converts the pyruvic acid to ethyl alcohol and carbon dioxide. – Lactic acid fermentation converts the pyruvic acid to lactic acid.
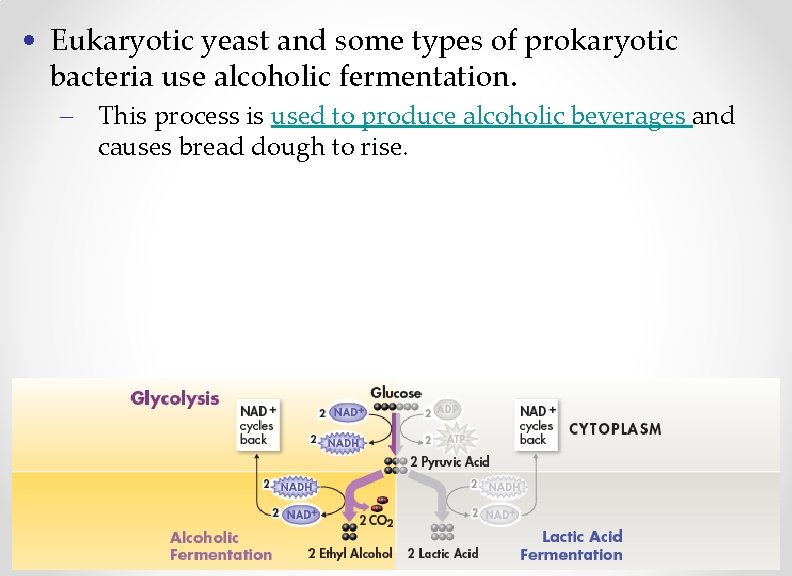
• Eukaryotic yeast and some types of prokaryotic bacteria use alcoholic fermentation. – This process is used to produce alcoholic beverages and causes bread dough to rise.
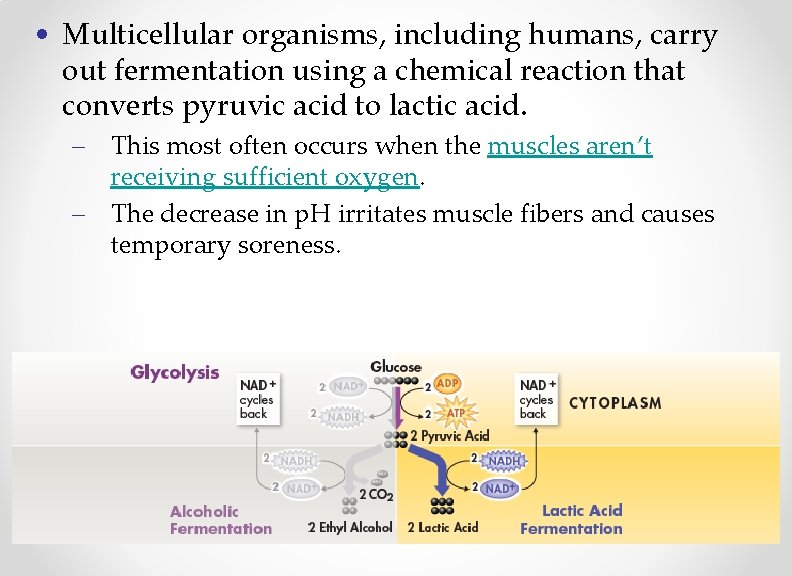
• Multicellular organisms, including humans, carry out fermentation using a chemical reaction that converts pyruvic acid to lactic acid. – This most often occurs when the muscles aren’t receiving sufficient oxygen. – The decrease in p. H irritates muscle fibers and causes temporary soreness.

Movement to Mitochondria • Before the next stage can begin, pyruvic acid must first be transported inside the mitochondria. • Pyruvic acid is combined with an enzyme called Coenzyme A. – This enzyme helps with the transportation of pyruvic acid into the mitochondria.
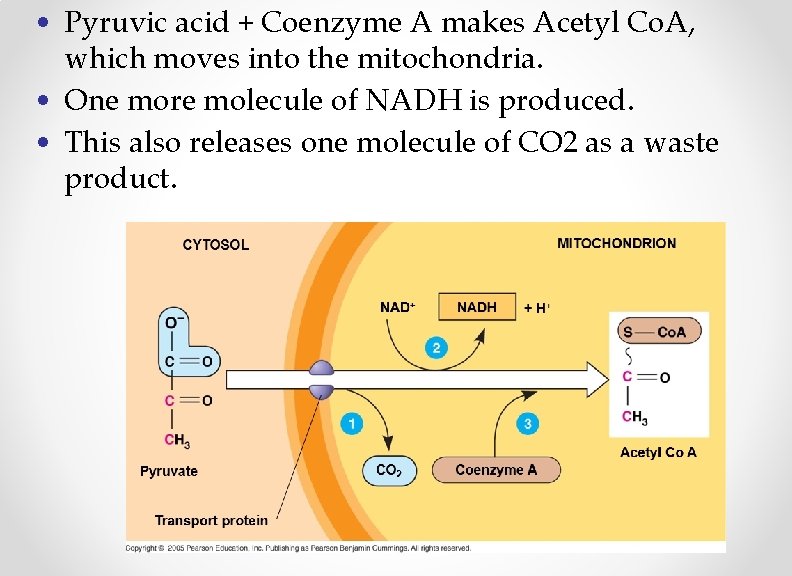
• Pyruvic acid + Coenzyme A makes Acetyl Co. A, which moves into the mitochondria. • One more molecule of NADH is produced. • This also releases one molecule of CO 2 as a waste product.
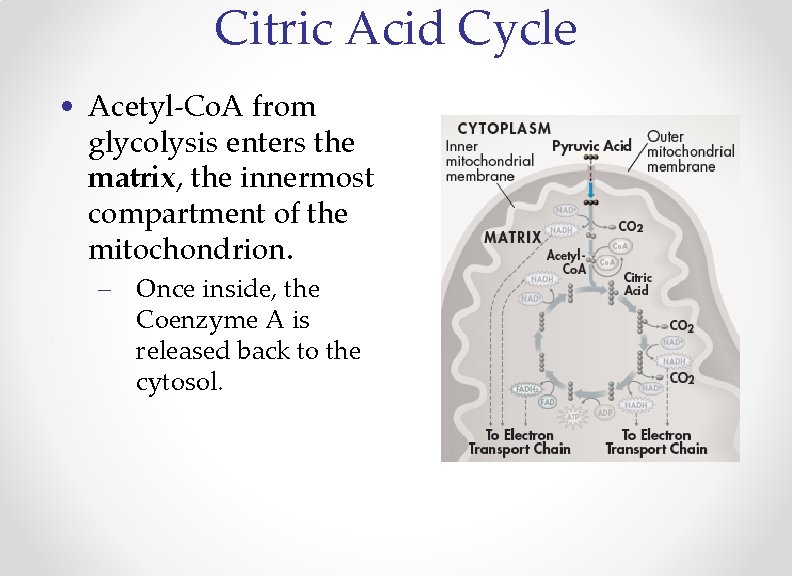
Citric Acid Cycle • Acetyl-Co. A from glycolysis enters the matrix, the innermost compartment of the mitochondrion. – Once inside, the Coenzyme A is released back to the cytosol.
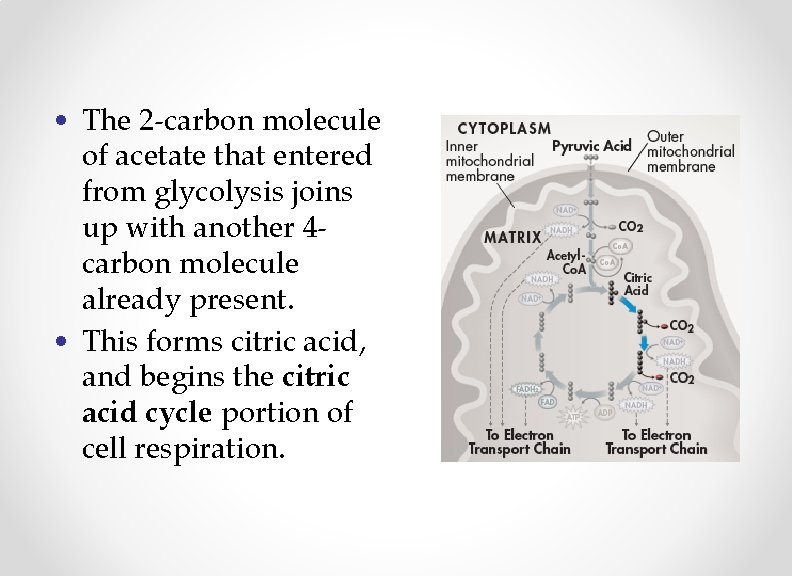
• The 2 -carbon molecule of acetate that entered from glycolysis joins up with another 4 carbon molecule already present. • This forms citric acid, and begins the citric acid cycle portion of cell respiration.
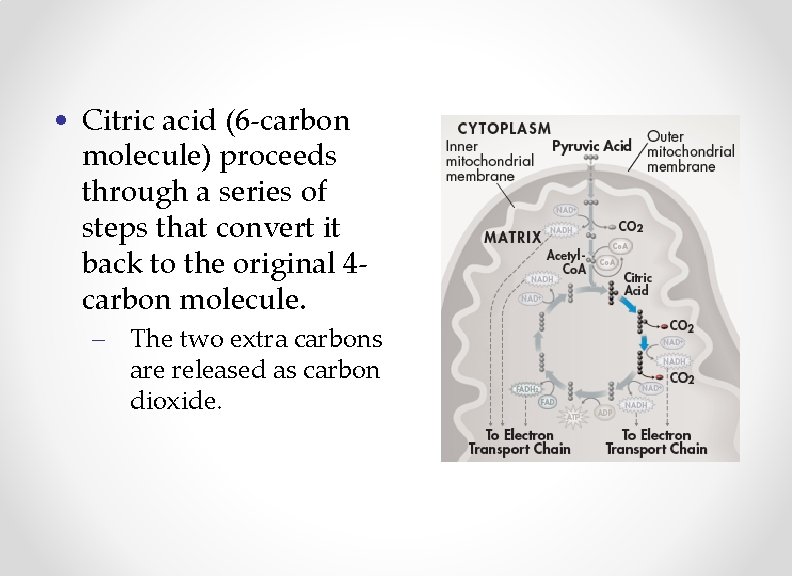
• Citric acid (6 -carbon molecule) proceeds through a series of steps that convert it back to the original 4 carbon molecule. – The two extra carbons are released as carbon dioxide.
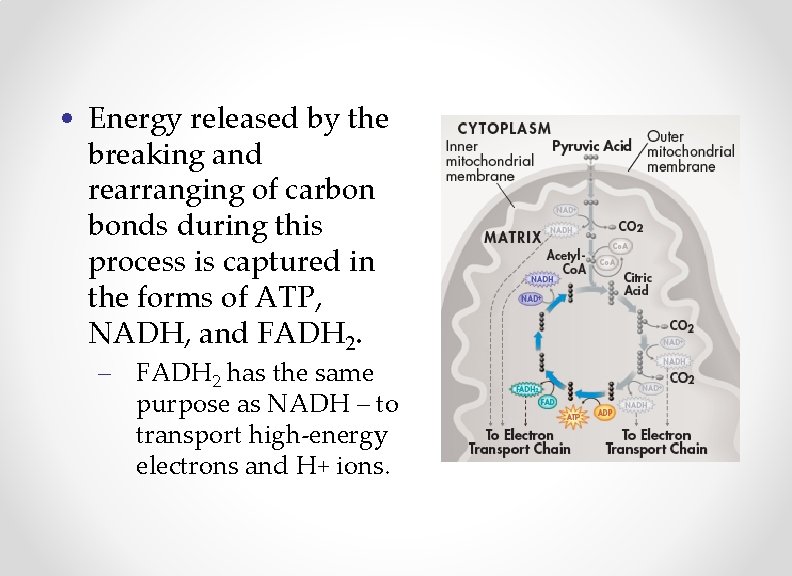
• Energy released by the breaking and rearranging of carbon bonds during this process is captured in the forms of ATP, NADH, and FADH 2. – FADH 2 has the same purpose as NADH – to transport high-energy electrons and H+ ions.
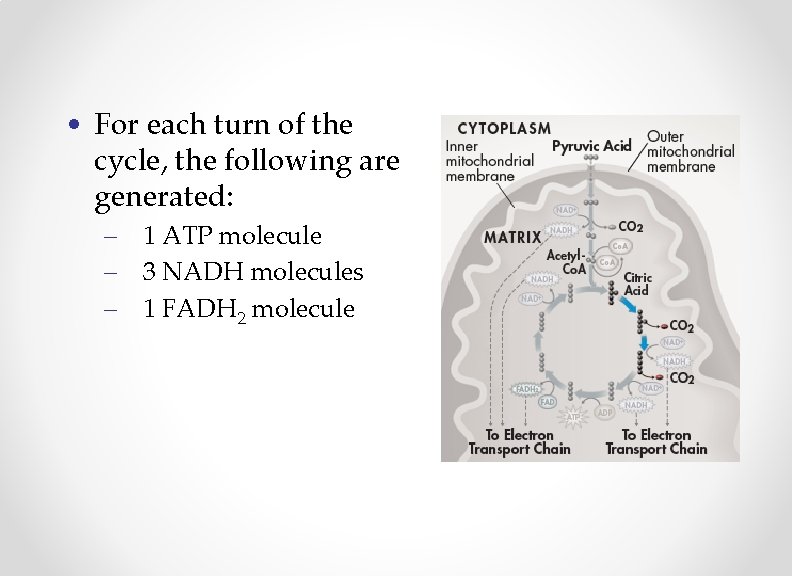
• For each turn of the cycle, the following are generated: – 1 ATP molecule – 3 NADH molecules – 1 FADH 2 molecule
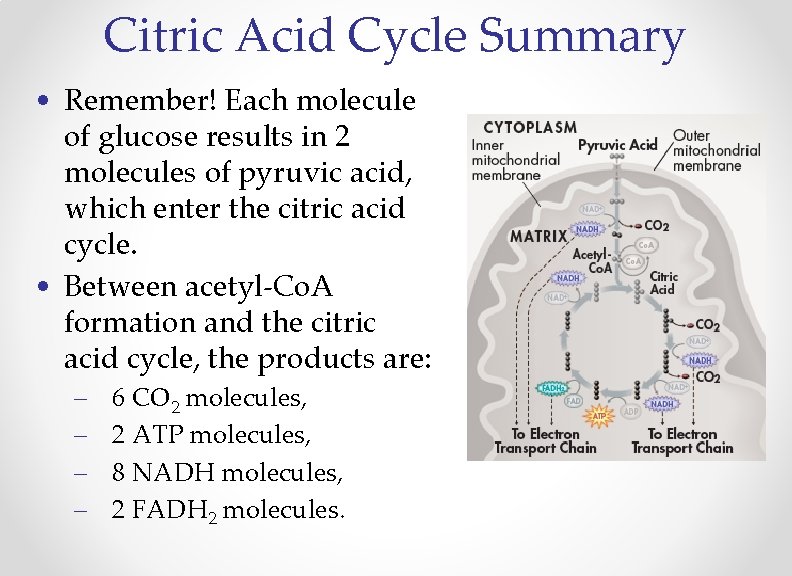
Citric Acid Cycle Summary • Remember! Each molecule of glucose results in 2 molecules of pyruvic acid, which enter the citric acid cycle. • Between acetyl-Co. A formation and the citric acid cycle, the products are: – – 6 CO 2 molecules, 2 ATP molecules, 8 NADH molecules, 2 FADH 2 molecules.

Electron Transport Chain • The electron transport chain occurs in the inner membrane of the mitochondria. – This part of cell respiration utilizes all of the molecules of NADH and FADH 2 that were generated in glycolysis and the citric acid cycle.
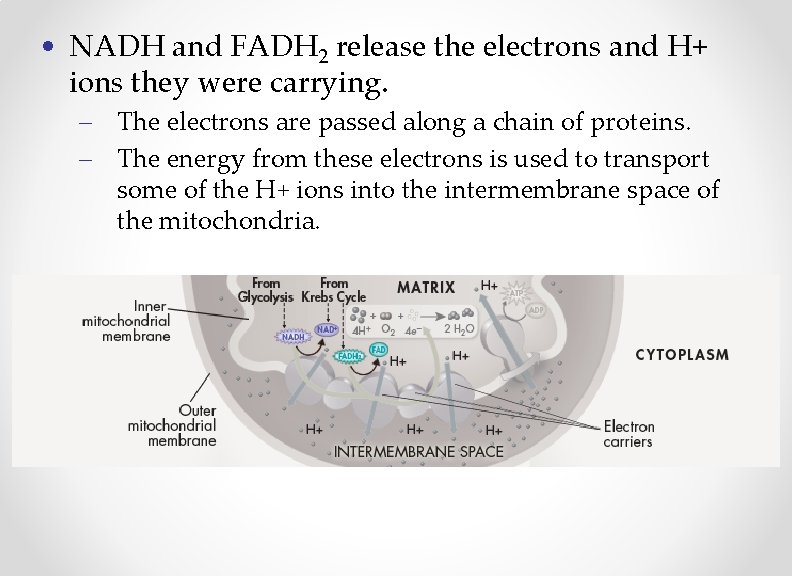
• NADH and FADH 2 release the electrons and H+ ions they were carrying. – The electrons are passed along a chain of proteins. – The energy from these electrons is used to transport some of the H+ ions into the intermembrane space of the mitochondria.
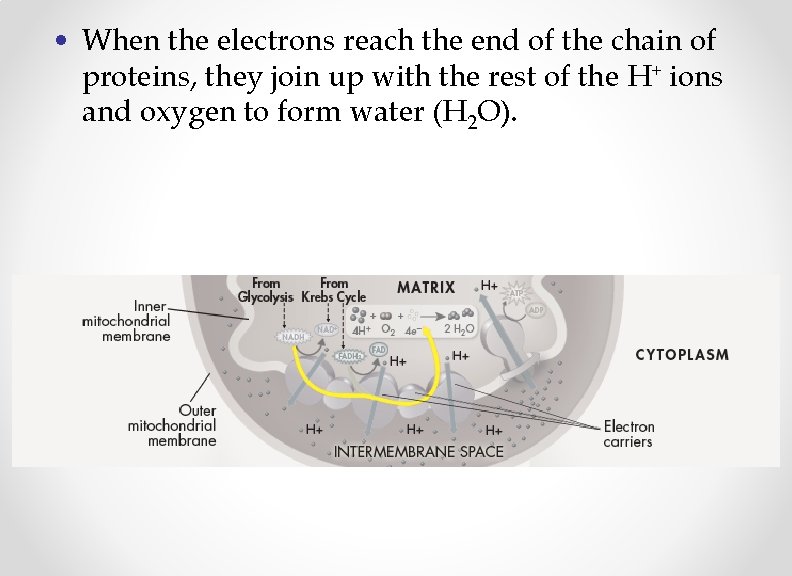
• When the electrons reach the end of the chain of proteins, they join up with the rest of the H+ ions and oxygen to form water (H 2 O).
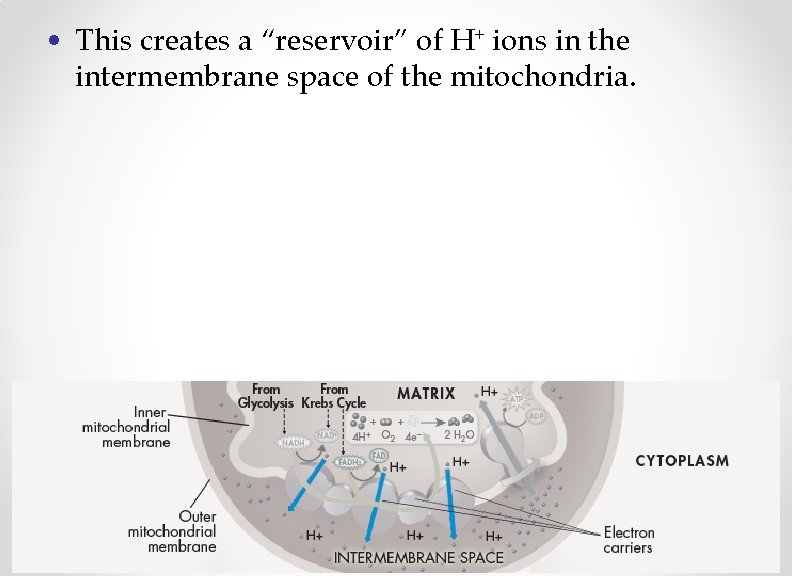
• This creates a “reservoir” of H+ ions in the intermembrane space of the mitochondria.
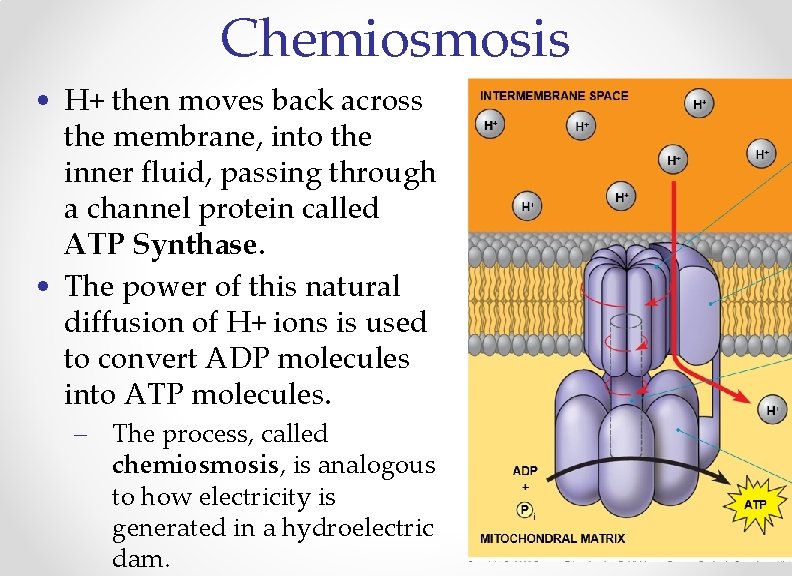
Chemiosmosis • H+ then moves back across the membrane, into the inner fluid, passing through a channel protein called ATP Synthase. • The power of this natural diffusion of H+ ions is used to convert ADP molecules into ATP molecules. – The process, called chemiosmosis, is analogous to how electricity is generated in a hydroelectric dam.
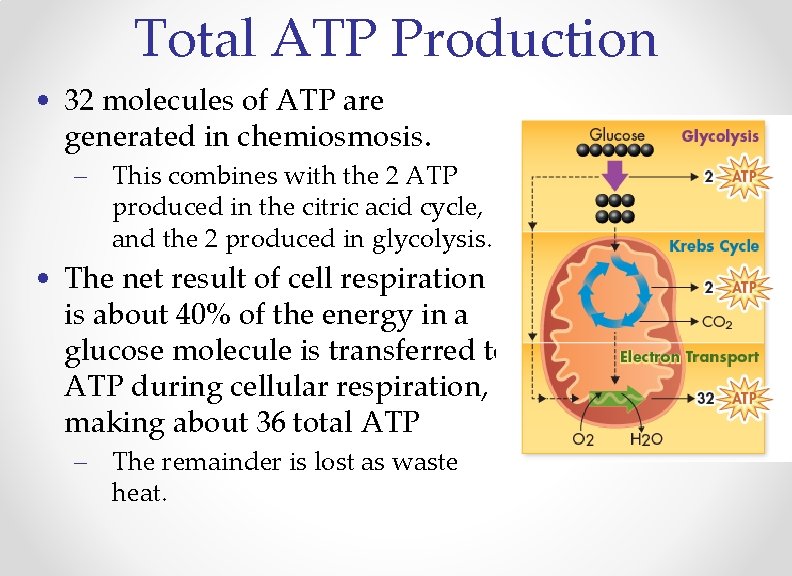
Total ATP Production • 32 molecules of ATP are generated in chemiosmosis. – This combines with the 2 ATP produced in the citric acid cycle, and the 2 produced in glycolysis. • The net result of cell respiration is about 40% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 36 total ATP – The remainder is lost as waste heat.
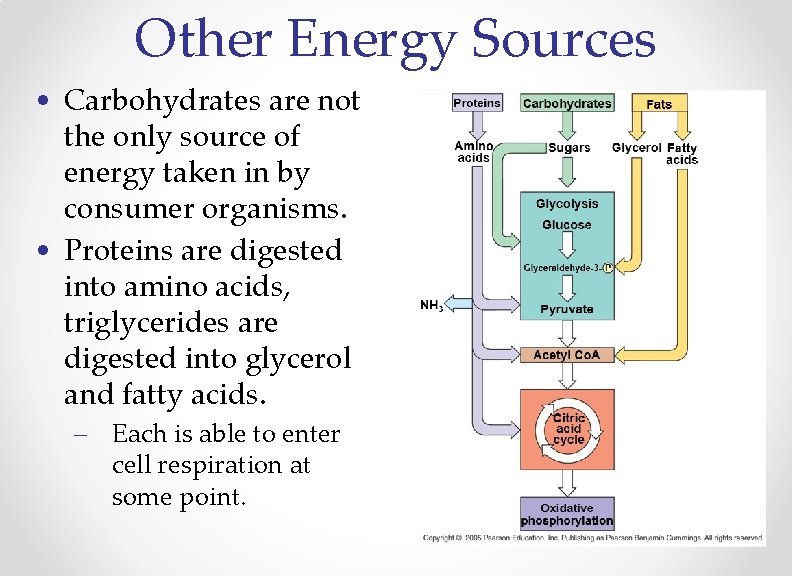
Other Energy Sources • Carbohydrates are not the only source of energy taken in by consumer organisms. • Proteins are digested into amino acids, triglycerides are digested into glycerol and fatty acids. – Each is able to enter cell respiration at some point.
Cell Respiration and Evolution • Glycolysis occurs in nearly all organisms, so it probably evolved in ancient prokaryotes before there was oxygen in the atmosphere. • The endosymbiosis theory states that the mitochondria of eukaryotes at one point was an independent species of prokaryotic bacteria that was absorbed by a eukaryotic cell through endocytosis.

Origin of Mitochondria • Mitochondria are unlike any other organelle in that they only originate from other mitochondria. – The mitochondria present in each of your cells all originated from the ones in the egg cell provided by your mother. – Sperm only provide DNA.
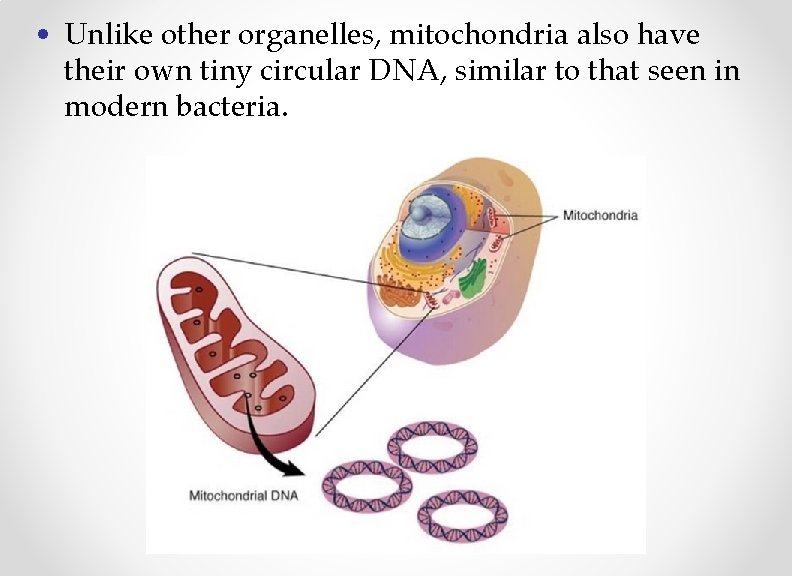
• Unlike other organelles, mitochondria also have their own tiny circular DNA, similar to that seen in modern bacteria.
- Slides: 35