Cell Membrane Transport Dr Ola Tork MD Physiology
Cell Membrane Transport Dr. Ola Tork MD. Physiology
Introduction n Interstitial fluid q Surrounds cells q Contained within tissues. q Part of the extra-cellular water compartment q Derived from blood plasma q Contains amino acids, vitamins, hormones, salts, waste products, etc. n Cells must be able to transport materials across the cell membrane q Movement of materials is controlled by the plasma membrane, that is selectively permeable so that it allows only certain materials pass in and out of the cell to maintain differences between ICF and ECF.
Mechanisms of transport across cell membrane are: n Passive transport (Diffusion) n Active transport n Bulk transport
Factors Affecting the Direction of Transport n The cell membrane is. q Impermeable to most water-soluble substances (substances that dissolve in water) q Closely controls passage of materials in and out of the cell. n Passive Transport versus Active Transport q Passive Transport 1) Movement of substances through the membrane without the use of energy from the cell is a physical or passive process. 2) Does not require ATP q Includes simple diffusion, facilitated diffusion, osmosis, and filtration. q Active Transport 1) Movement of material through the membrane that requires metabolic energy (ATP) is called an active physiological process. q Includes Primary and Secondary Active Transport
Driving Forces Acting on Molecules n Chemical driving force q Difference in energy due to a concentration gradient that causes a molecule to move from high to low concentration n Electrical driving force q Difference in energy due to a separation of charge that acts to move ions from high energy to low energy n Electrochemical driving force q Sum of the chemical and electrical driving forces
Simple diffusion n Routs of diffusion: 1. Through lipid bilayer: the rate of diffusion is directly proportional to lipid solubility of substances: A. Lipid soluble substances(O 2, N 2…) B. Water molecules : as they are small & have high kinetic energy C. Lipid insoluble : if they are small and unchrged
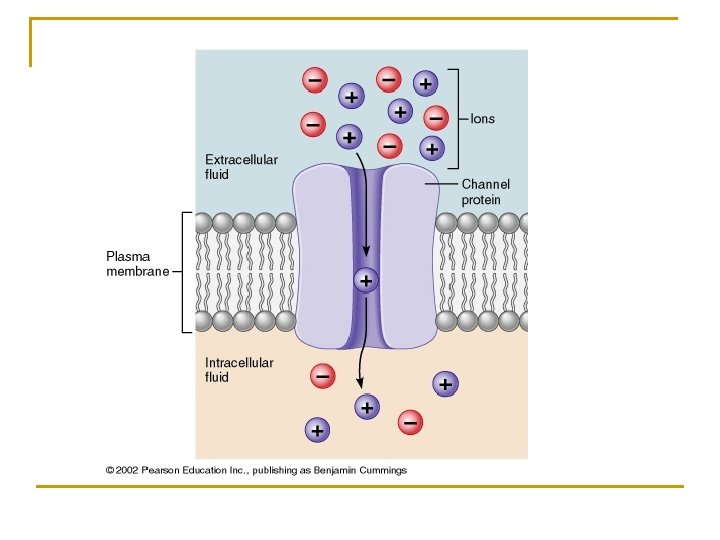
n n n 1. 2. Through protein channels: For transport of ions mainly They have: Selective permeability Gates : their opening and closing are controlled by Ø Voltage gating : Ø Ligand gating: acetylecholine receptors
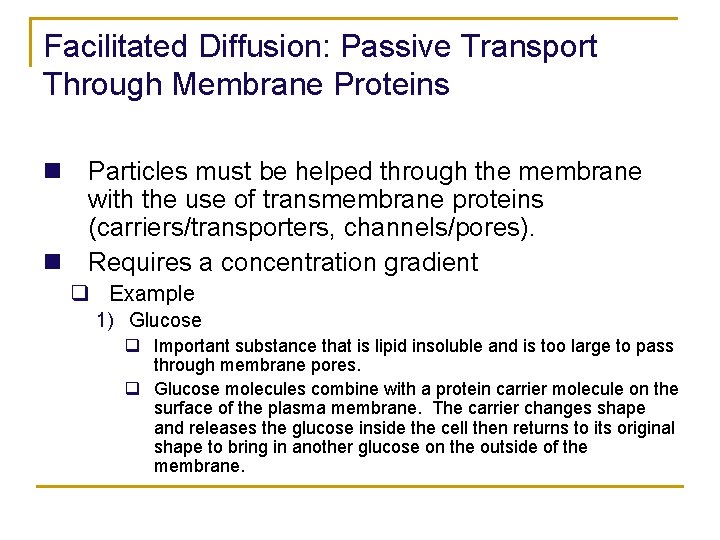
Facilitated Diffusion: Passive Transport Through Membrane Proteins n Particles must be helped through the membrane with the use of transmembrane proteins (carriers/transporters, channels/pores). n Requires a concentration gradient q Example 1) Glucose q Important substance that is lipid insoluble and is too large to pass through membrane pores. q Glucose molecules combine with a protein carrier molecule on the surface of the plasma membrane. The carrier changes shape and releases the glucose inside the cell then returns to its original shape to bring in another glucose on the outside of the membrane.

Transport Proteins in Facilitated Diffusion q Carriers 1) A transmembrane protein that binds to a molecule on one side of the membrane 2) Conformational change q The carrier “flips” to bring the transported molecule to the other side of the membrane 3) Transport is limited by the number of carriers available on the membrane.
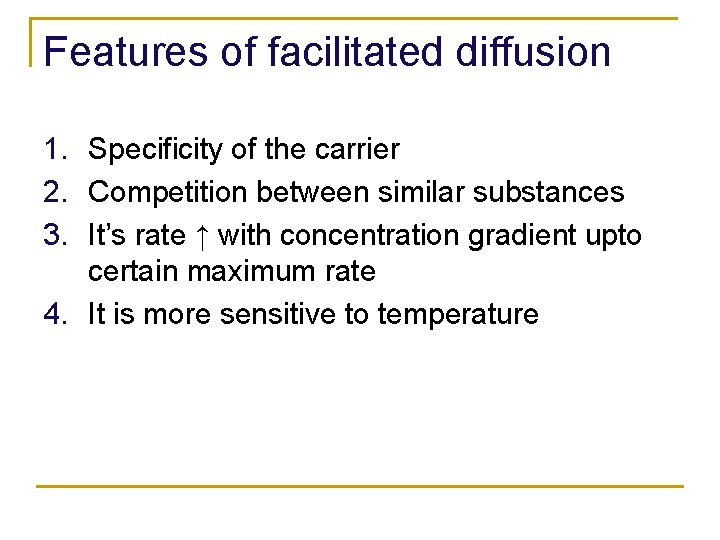
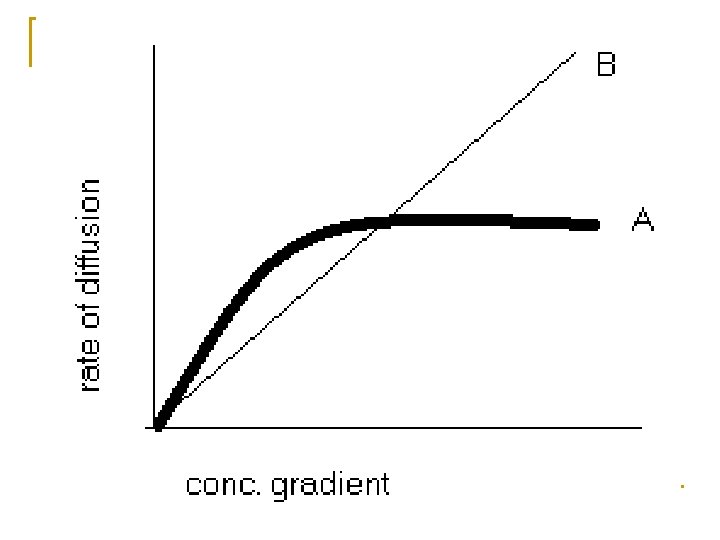
Features of facilitated diffusion 1. Specificity of the carrier 2. Competition between similar substances 3. It’s rate ↑ with concentration gradient upto certain maximum rate 4. It is more sensitive to temperature

Osmosis: Passive Transport of Water Across Membranes n The flow of water across a selectively permeable membrane q Always from an area of high water concentration to an area of low water concentration. q A special case of diffusion of water across a selectively permeable membrane, such as the plasma membrane. 1) A semi-permeable membrane is freely permeable to water but not to solutes. n It is a very important process because water is found throughout cells and extra-cellular areas of the body.
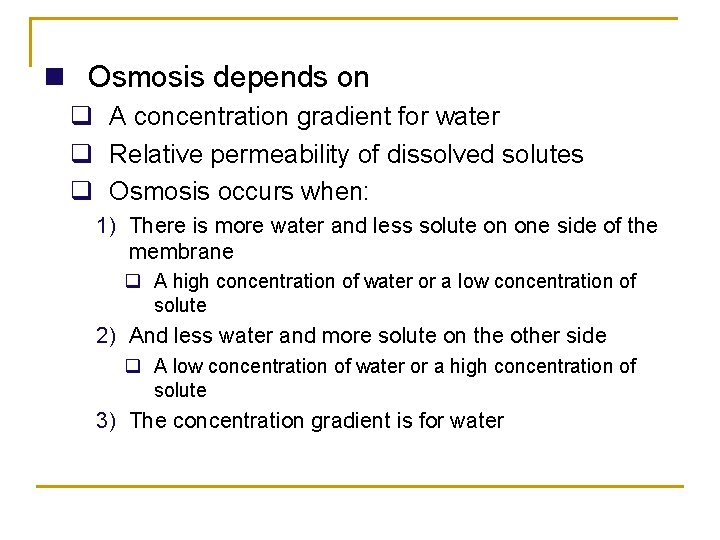
n Osmosis depends on q A concentration gradient for water q Relative permeability of dissolved solutes q Osmosis occurs when: 1) There is more water and less solute on one side of the membrane q A high concentration of water or a low concentration of solute 2) And less water and more solute on the other side q A low concentration of water or a high concentration of solute 3) The concentration gradient is for water

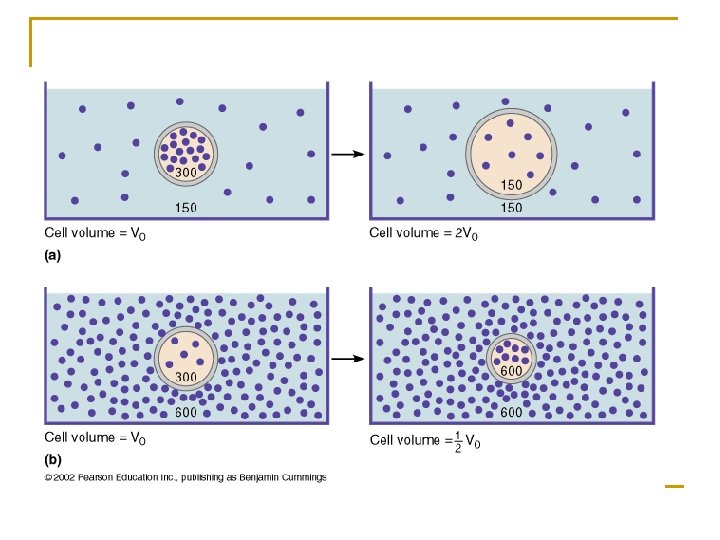
q Osmolarity 1) Total solute concentration 2) Unit is osmole (Osm) or milliosmole (m. Osm) q Normal osmolarity (concentration) of body fluids is 300 m. Osm q Total solute concentration is 300 milliosmoles per liter 3) Depends on the total concentration of dissolved solutes 4) Example q 150 m. Osm Na. Cl q Dissolved in water the molecule separated into two particles, so osmolarity is doubled, 300 m. Osm

Tonicity n It is the osmolaritity of a solution relative to the osmolarity of plasma
Types of solutions 1) Iso-tonic q Same osmolarity as plasma 2) Hyper-tonic q Solution has greater osmolarity than plasma 3) Hypo-tonic q Solution has lower osmolarity than plasma
n Isotonic q A solution that has the same concentration of solute (osmotic pressure) as body fluids. q Fluid surrounding a cell has the same concentration of solute as that inside of the cell. q No osmosis occurs. n Hypotonic q A solution that has a lower concentration of solute (osmotic pressure) than body fluids. q Hypotonic extra-cellular fluid has a lower concentration of solute than the concentration inside cell and causes water to move into the cell following its concentration gradient (more water outside, less inside). q Too much water moving into the cell membrane may cause the cell to burst.

n Hypertonic q A solution has a higher concentration of solute (osmotic pressure) than the concentration found in body fluids. q Hypertonic extra-cellular fluid will cause water to leave the cell following its concentration gradient (more water inside, less outside) producing a shrunken or crenated cell.

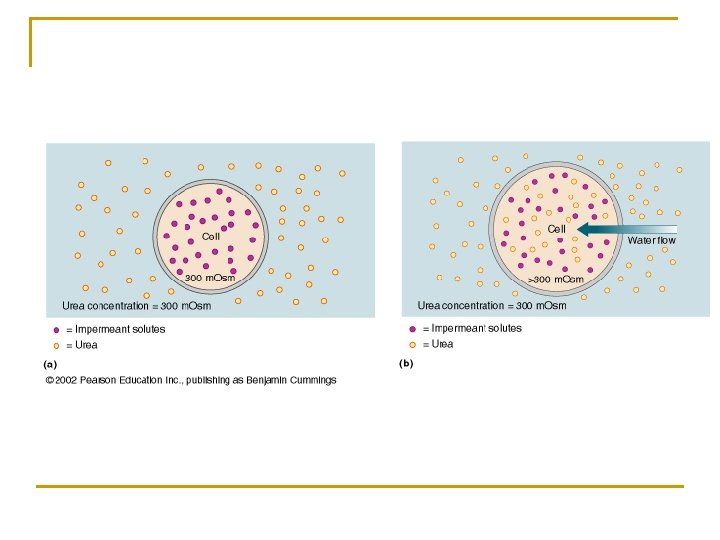
n Isotonic saline solution q A solution that is. 9% saline because the body's red blood cells are. 9% salt or Na. Cl. q Therefore, when an isotonic saline solution is introduced into the body, fluid equilibrium will be maintained. n Remember: q The key to understanding the above terms and process is to understand that hyper and hypo refer to the solute in the solution, not to the water. q Water will move toward the greater amount of solute because the concentration of water there is less.

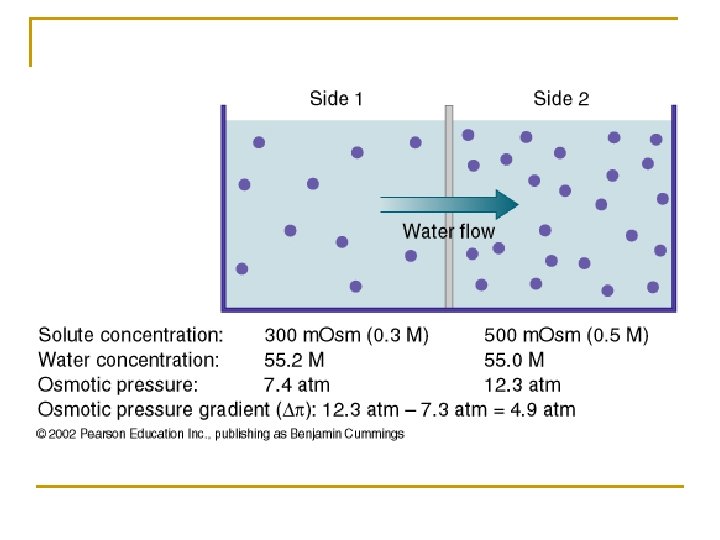
n Osmotic Pressure (π) q The membrane is selectively permeable in that it does not allow the solute to pass, it is not permeable to certain molecules, particles, or solute. 1) Remember that high solute concentration means low water concentration (requires more water to reach equilibrium) and low solute concentration means high water concentration (requires water to leave to reach equilibrium). q Osmosis will continue to occur or the water will continue to move until: 1) Equilibrium for water is reached so that the concentration of water and solute is equal on each side of the membrane.

1) Osmotic pressure stops the movement of water. q Osmotic pressure is the amount of pressure required to prevent further water movement. q The ability of osmosis to generate enough pressure to lift a volume of water. q A potential pressure due to the presence of non-diffusible solute particles. q The greater the amount of non-diffusible solute, the greater the gradient attracting water across the membrane and the greater the osmotic pressure produced. 2) Example q Na. Cl is a very osmotically active particle because when it dissociates it produces two ions, or double the osmotic activity q Water movement changes the volume of water in the container or cell.
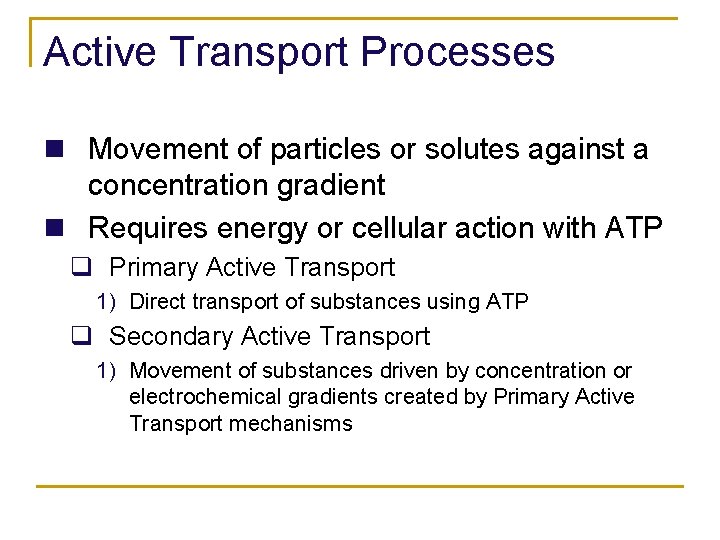
Active Transport Processes n Movement of particles or solutes against a concentration gradient n Requires energy or cellular action with ATP q Primary Active Transport 1) Direct transport of substances using ATP q Secondary Active Transport 1) Movement of substances driven by concentration or electrochemical gradients created by Primary Active Transport mechanisms
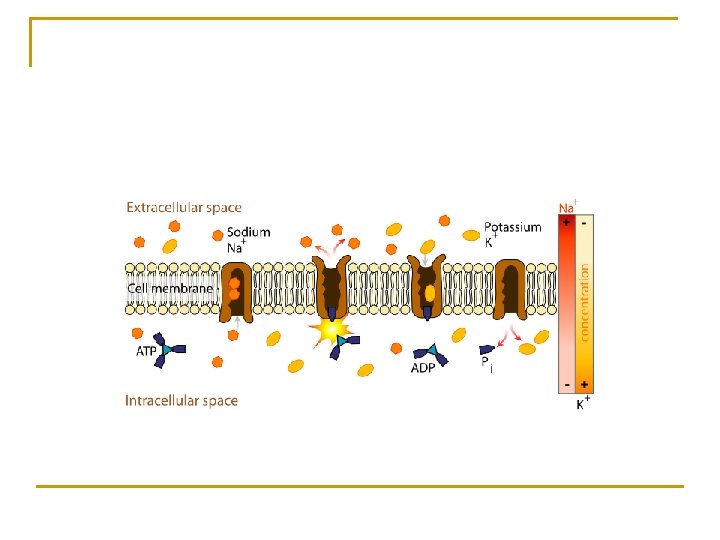
Primary Active Transport n Solute pumping q Pump or protein carrier 1) An enzyme-like protein carrier that pumps or carries solutes such as ions of sodium, potassium, and calcium, into or out of the cell against their concentration gradients. q ATPase 1) The enzyme on the protein carrier or pump that catalyzes the breakdown or phosphorylation of ATP producing energy that drives the pump. q This action may require up to 40% of a cell’s supply of ATP
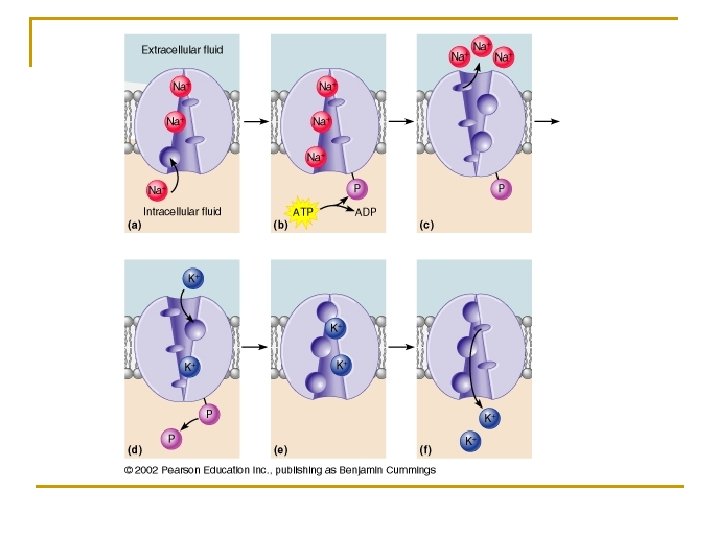
q Sodium-potassium pump (Na+/K+ ATPase Pump) 1) Maintains the resting membrane potential of nerve and muscle cells 2) Sodium q Primary extra-cellular ion that is constantly “leaking” into cells. 3) Potassium q Primary intracellular ion that is constantly “leaking” out of cells. 4) The sodium/potassium pump constantly pumps 3 sodium ions out and 2 potassium ions into the cell, maintaining the relative negativity inside the cell. 5) All cells have a negative charge inside because of this mechanism.
Solute Pumping to Maintain the Membrane Potential n Pumps q Transport proteins that use energy from ATP hydrolysis to transport specific molecules against the electrochemical gradient across a membrane n Sodium-Potassium pump (Na+/K+ ATPase Pump) q Transports Na+/K+ ions in opposite directions across cell membranes q Move 3 Na+ ions out of the cell for every 2 K+ ions into cell q Specific for Na+/K+ and unidirectional q Phosphorylation of the pump protein causes a conformational change that turns the binding sites outward to expel Na+ q Also decreases affinity for Na+ and increases its affinity for K+ q Critical in maintaining resting membrane potential for nerve and muscle impulse conduction
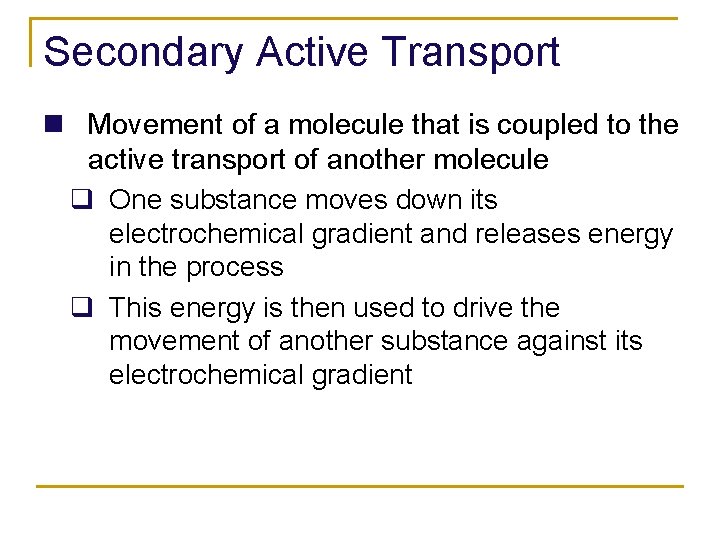
Secondary Active Transport n Movement of a molecule that is coupled to the active transport of another molecule q One substance moves down its electrochemical gradient and releases energy in the process q This energy is then used to drive the movement of another substance against its electrochemical gradient
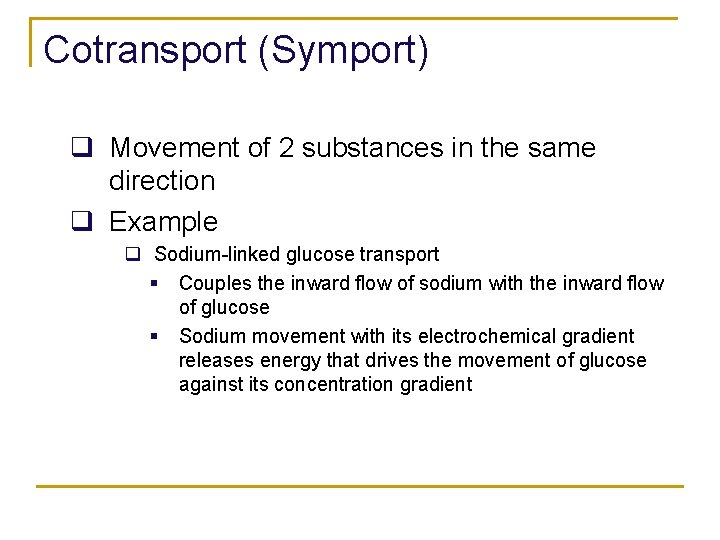
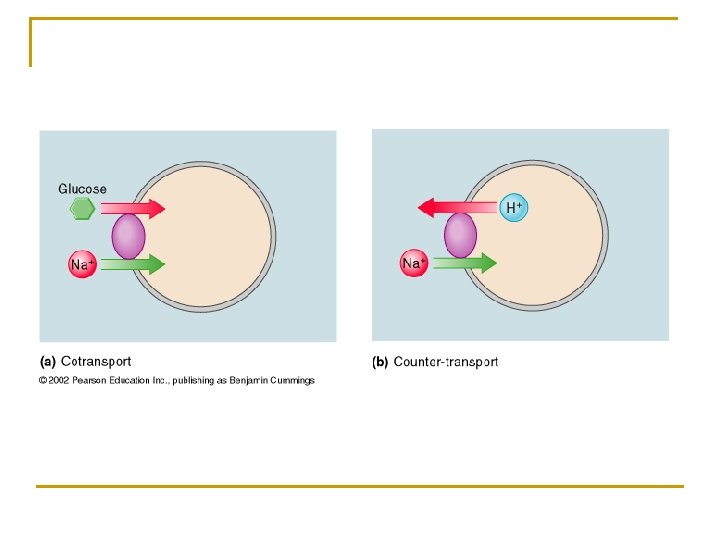
Cotransport (Symport) q Movement of 2 substances in the same direction q Example q Sodium-linked glucose transport § Couples the inward flow of sodium with the inward flow of glucose § Sodium movement with its electrochemical gradient releases energy that drives the movement of glucose against its concentration gradient
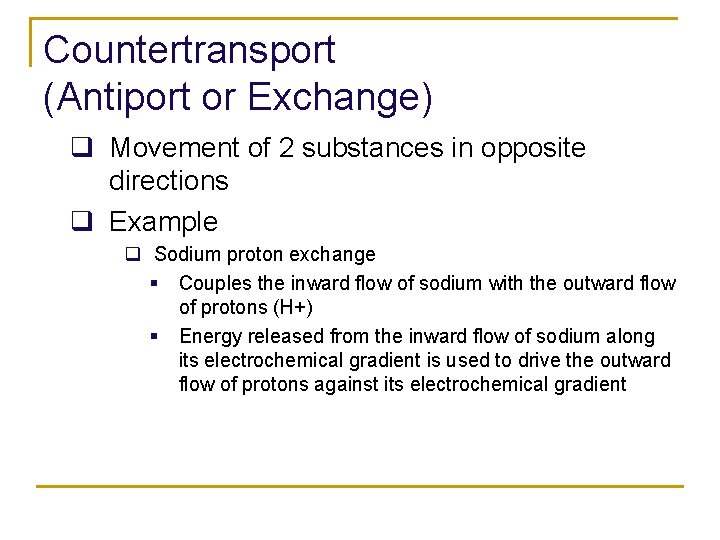
Countertransport (Antiport or Exchange) q Movement of 2 substances in opposite directions q Example q Sodium proton exchange § Couples the inward flow of sodium with the outward flow of protons (H+) § Energy released from the inward flow of sodium along its electrochemical gradient is used to drive the outward flow of protons against its electrochemical gradient

Bulk transport n Endocytosis: n Exocytosis :
Endocytosis n Endocytosis is the process by which cells ingest materials. The cellular membrane folds around the desired materials outside the cell. The ingested particle is trapped within a pouch, vacuole or inside the cytoplasm. Often enzymes from lysosomes are then used to digest the molecules absorbed by this process.

Types : n pinocytosis and phagocytosis. n In pinocytosis, cells engulf liquid particles (in humans this process occurs in the small intestine, cells there engulf fat droplets) n In phagocytosis, cells engulf solid particles.
Exocytosis n Exocytosis is the process by which cells excrete waste and other large molecules from the protoplasm
- Slides: 41