Cell Membrane Homeostasis Label Add your notes Cell

Cell Membrane: Homeostasis

Label Add your notes Cell Membrane: Homeostasis Notes Topic Headings to the Left Hand margin

When a solid is dissolved in a liquid, or when two liquids are completely combined with each other, they form a solution. For example: Ocean water is a solution of water (96. 5%) and salt (3. 5%)
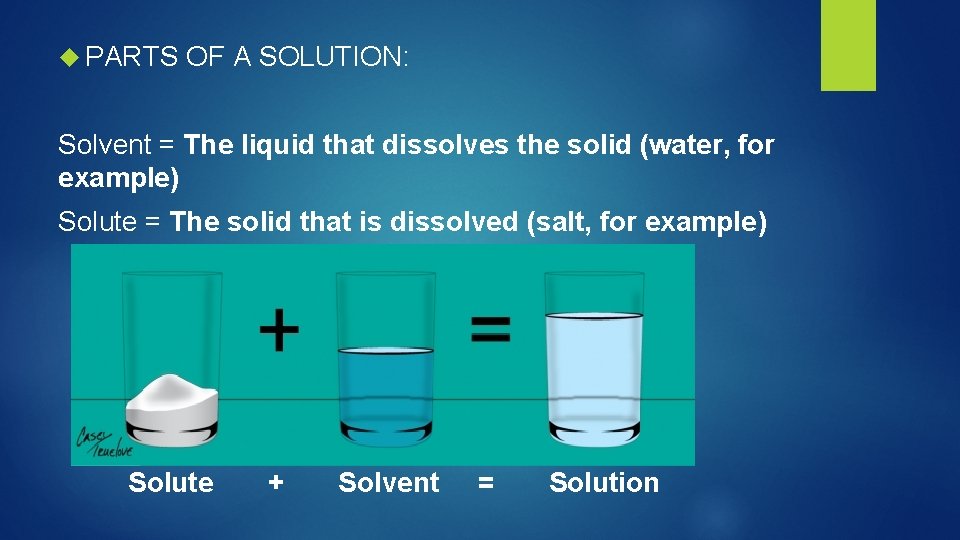
PARTS OF A SOLUTION: Solvent = The liquid that dissolves the solid (water, for example) Solute = The solid that is dissolved (salt, for example) Solute + Solvent = Solution
All cells exist in a solution of water and dissolved substances. There is a solution inside of them and a solution outside of them. Cells want the two solutions (inside and outside) to be balanced.
Homeostasis is a term that is used to both describe a healthy balance. For example, we can use this term to describe the successful survival of cells inside of an organism.
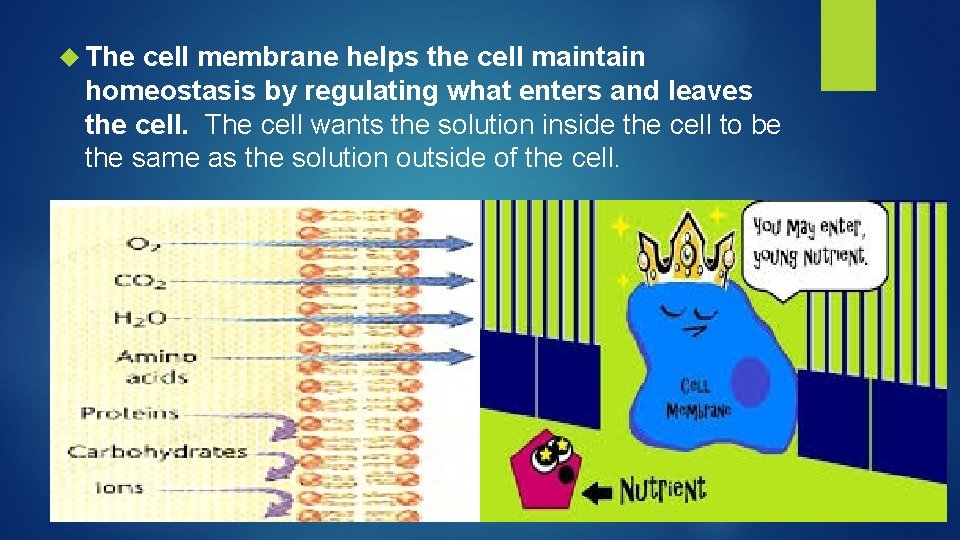
The cell membrane helps the cell maintain homeostasis by regulating what enters and leaves the cell. The cell wants the solution inside the cell to be the same as the solution outside of the cell.
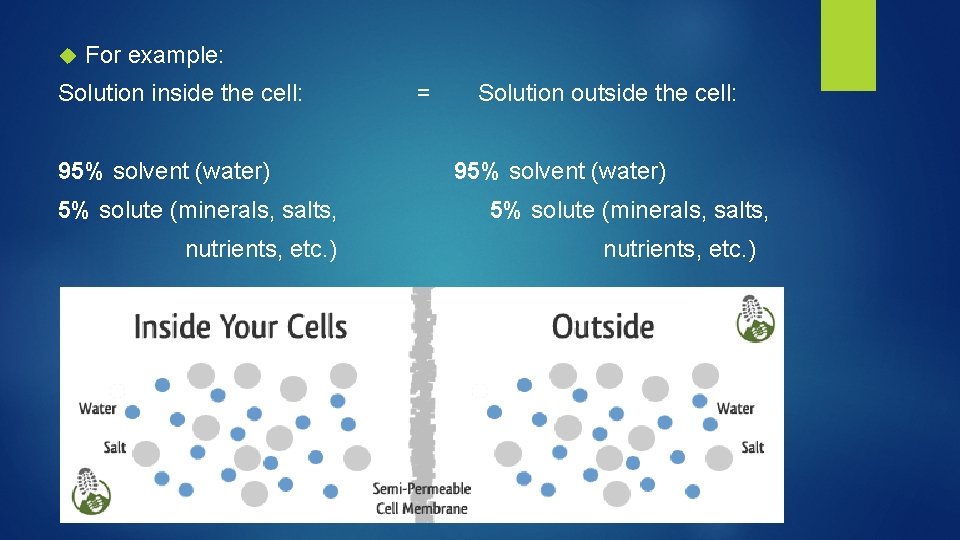
For example: Solution inside the cell: 95% solvent (water) 5% solute (minerals, salts, nutrients, etc. ) = Solution outside the cell: 95% solvent (water) 5% solute (minerals, salts, nutrients, etc. )
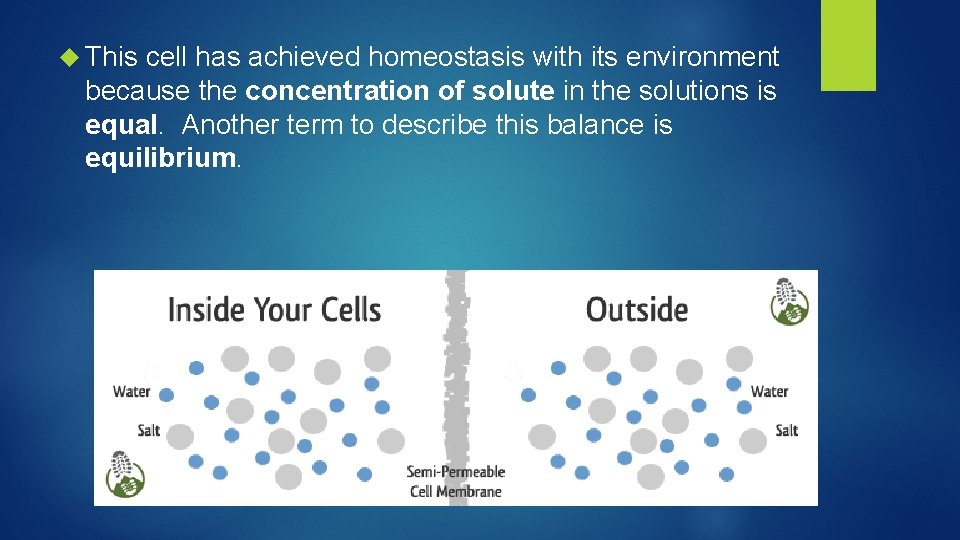
This cell has achieved homeostasis with its environment because the concentration of solute in the solutions is equal. Another term to describe this balance is equilibrium.
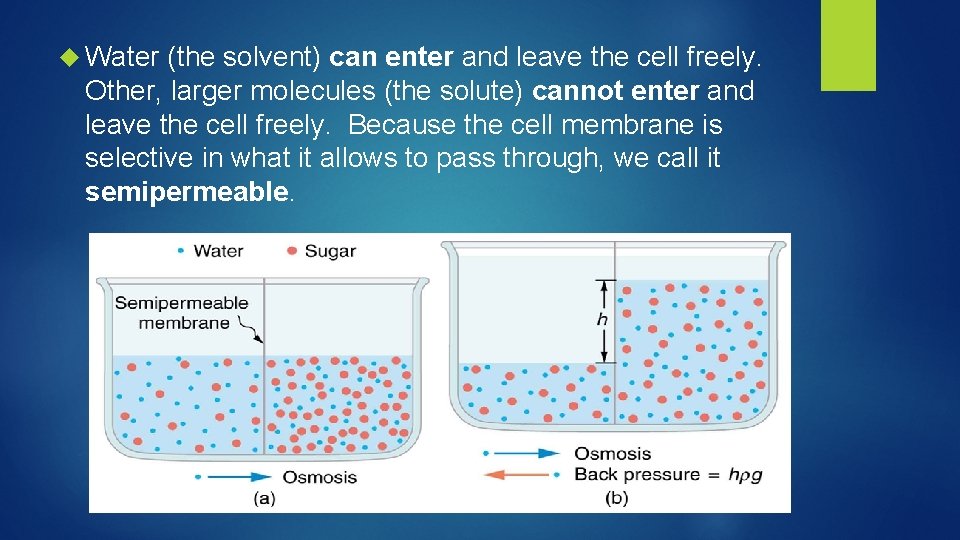
Water (the solvent) can enter and leave the cell freely. Other, larger molecules (the solute) cannot enter and leave the cell freely. Because the cell membrane is selective in what it allows to pass through, we call it semipermeable.
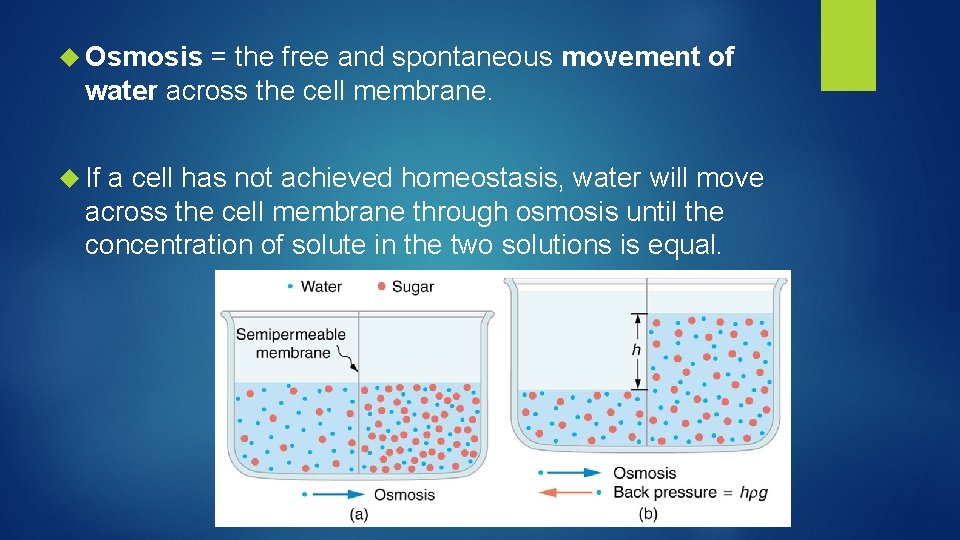
Osmosis = the free and spontaneous movement of water across the cell membrane. If a cell has not achieved homeostasis, water will move across the cell membrane through osmosis until the concentration of solute in the two solutions is equal.
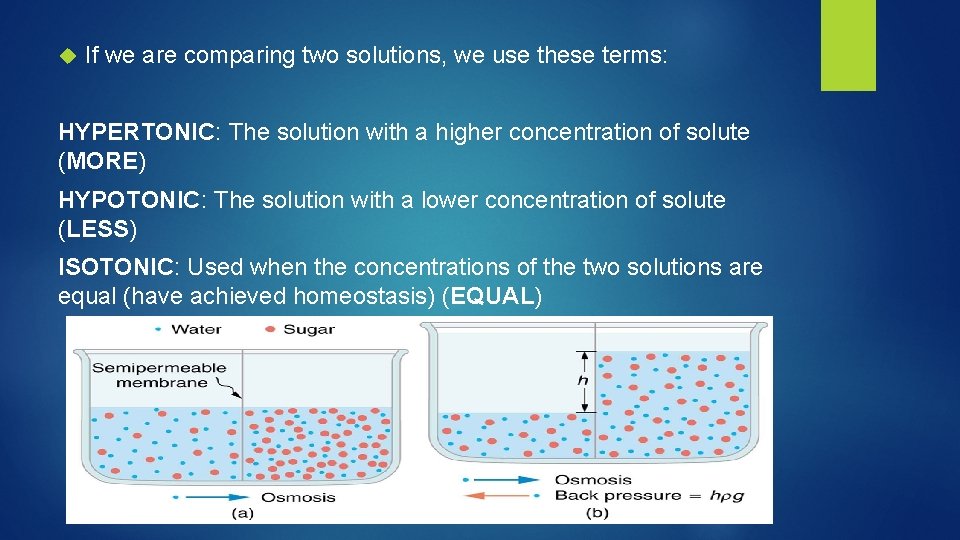
If we are comparing two solutions, we use these terms: HYPERTONIC: The solution with a higher concentration of solute (MORE) HYPOTONIC: The solution with a lower concentration of solute (LESS) ISOTONIC: Used when the concentrations of the two solutions are equal (have achieved homeostasis) (EQUAL)
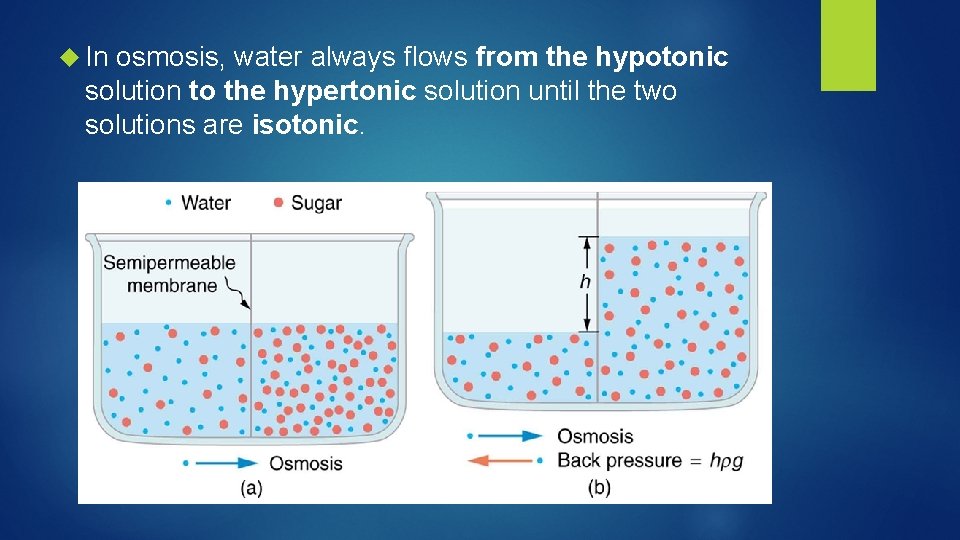
In osmosis, water always flows from the hypotonic solution to the hypertonic solution until the two solutions are isotonic.

Because water is allowed to move freely across the cell membrane, osmosis does not require energy. We call the movement of substances across the cell membrane that do not require energy passive transport.
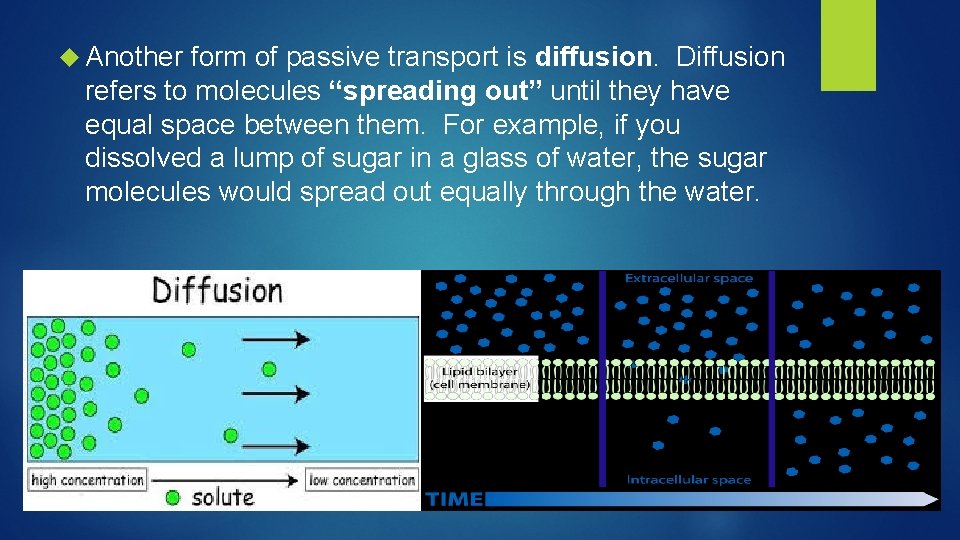
Another form of passive transport is diffusion. Diffusion refers to molecules “spreading out” until they have equal space between them. For example, if you dissolved a lump of sugar in a glass of water, the sugar molecules would spread out equally through the water.
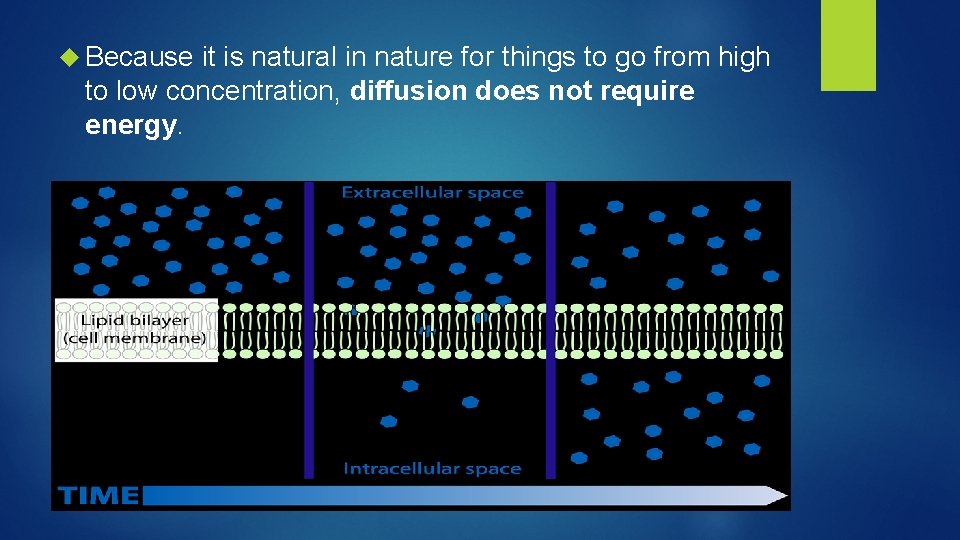
Because it is natural in nature for things to go from high to low concentration, diffusion does not require energy.
- Slides: 16