Cell Membrane and Solutions How do cells maintain
Cell Membrane and Solutions How do cells maintain homeostasis �Homeostasis – the process by which living things maintain a stable internal environment. �Can everything go in and out of a cell? �The Cell membrane regulates what enters and leave the cell.
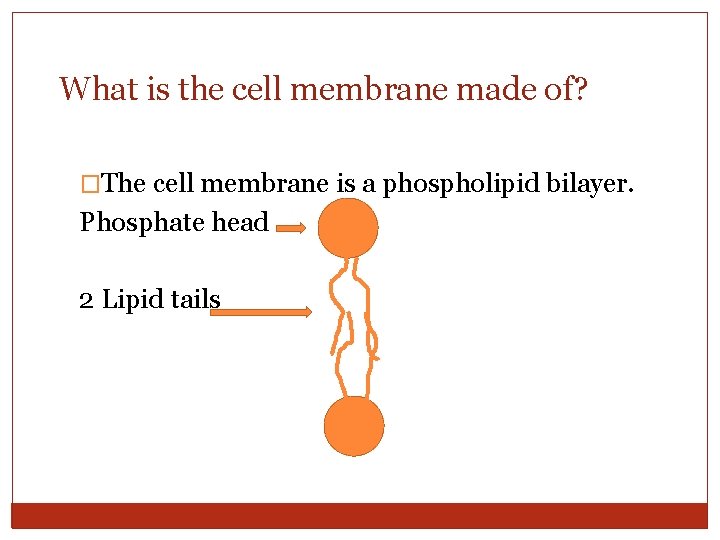
What is the cell membrane made of? �The cell membrane is a phospholipid bilayer. Phosphate head 2 Lipid tails
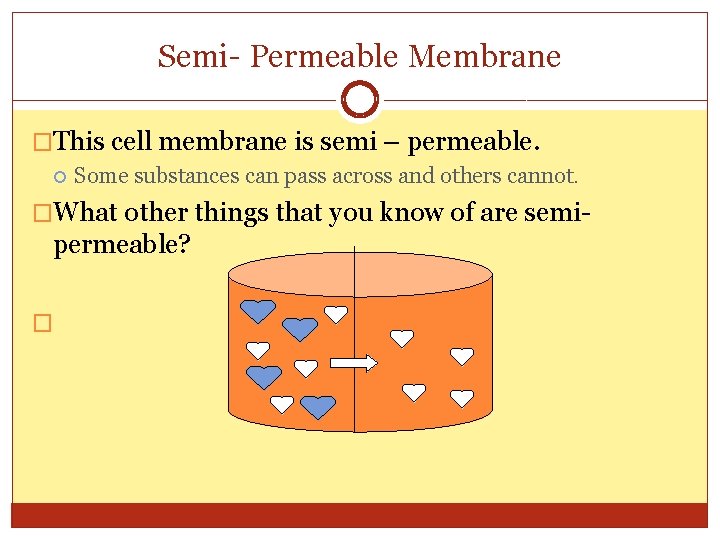
Semi- Permeable Membrane �This cell membrane is semi – permeable. Some substances can pass across and others cannot. �What other things that you know of are semi- permeable? �
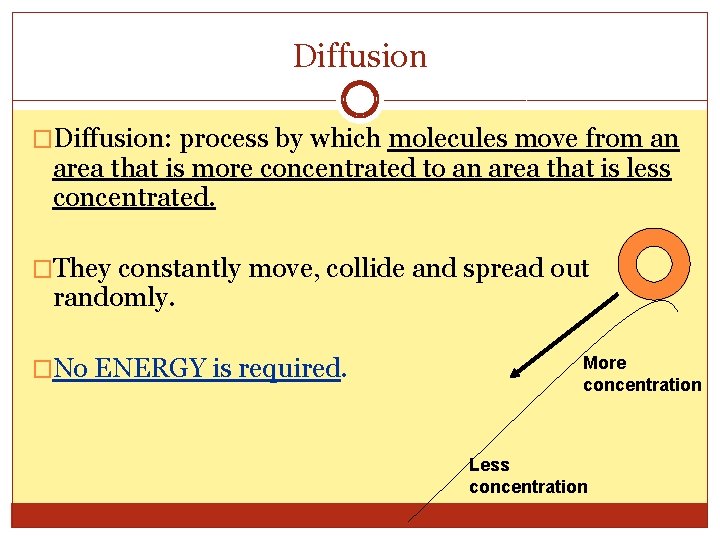
Diffusion �Diffusion: process by which molecules move from an area that is more concentrated to an area that is less concentrated. �They constantly move, collide and spread out randomly. �No ENERGY is required. More concentration Less concentration
Osmosis �Osmosis: the diffusion of water through a selectively permeable membrane. �Water moves from an area of greater water concentration (more pressure) to an area of lesser water concentration (less pressure). �Water passes across the membrane easily, but some solute molecules are too big to cross.
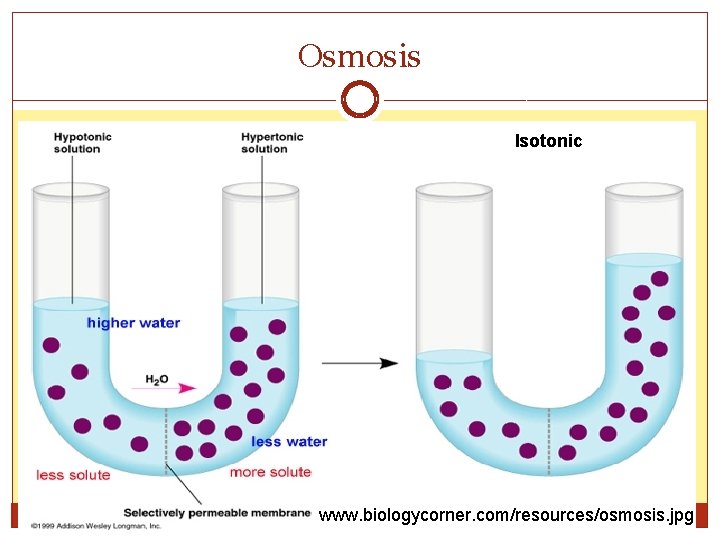
Osmosis Isotonic www. biologycorner. com/resources/osmosis. jpg
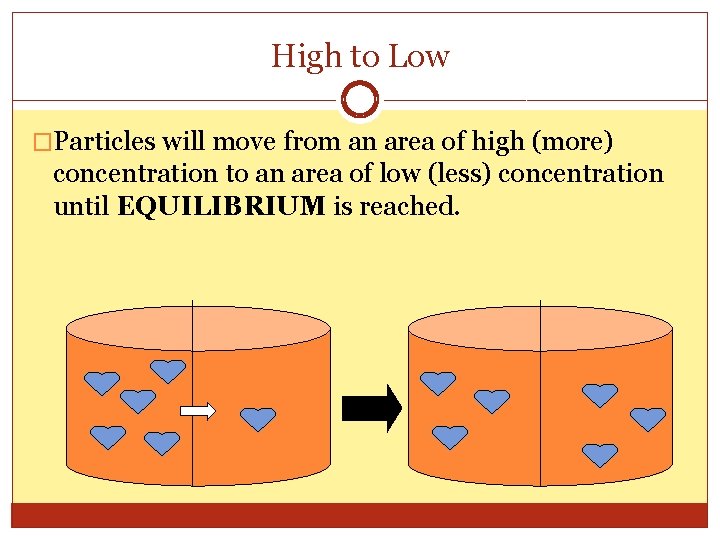
High to Low �Particles will move from an area of high (more) concentration to an area of low (less) concentration until EQUILIBRIUM is reached.
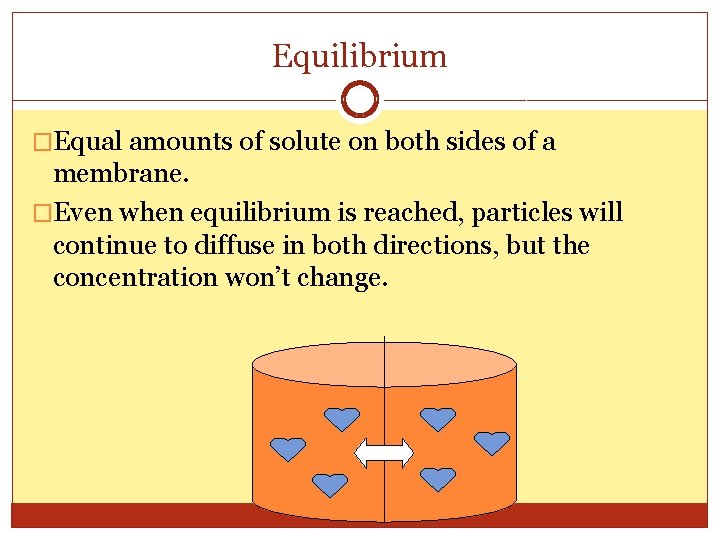
Equilibrium �Equal amounts of solute on both sides of a membrane. �Even when equilibrium is reached, particles will continue to diffuse in both directions, but the concentration won’t change.

What is it called when stuff dissolves in water? �Solute = Stuff that dissolves (Sugar) Gasses: oxygen, carbon dioxide Solids: Salts, sugars, amino acids, proteins �Solvent = What it dissolves in (Water) �A “Solution” is when the solute is evenly distributed throughout the solvent. �A “Suspension” is when the substance doesn’t dissolve. �Example –oil in water, salt in water
Measuring Concentration �The cytoplasm of cells is a solution of many things dissolved in water. Cytoplasm is mostly WATER! �The concentration of a solution is the mass of solute in a given volume of solvent. (mass/volume) �What is the concentration of 12 grams of salt in 3 liters of water? 12 g/3 L = 4 g/L

Types of Solutions �Isotonic solution: Same strength The solute concentration is the same inside and outside the cell. �Hypertonic solution: Above strength The solute concentration is higher in in the solution surrounding the cell than inside. �Hypotonic solution: Below strength The solute concentration is lower in the solution surrounding the cell than inside.
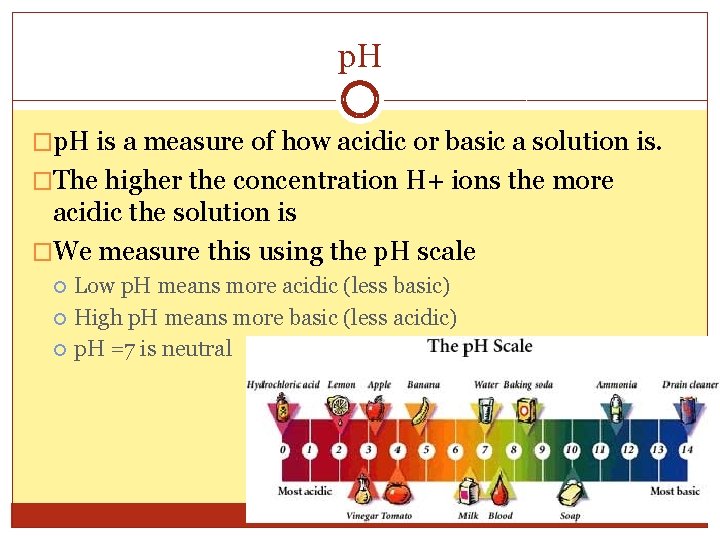
p. H �p. H is a measure of how acidic or basic a solution is. �The higher the concentration H+ ions the more acidic the solution is �We measure this using the p. H scale Low p. H means more acidic (less basic) High p. H means more basic (less acidic) p. H =7 is neutral
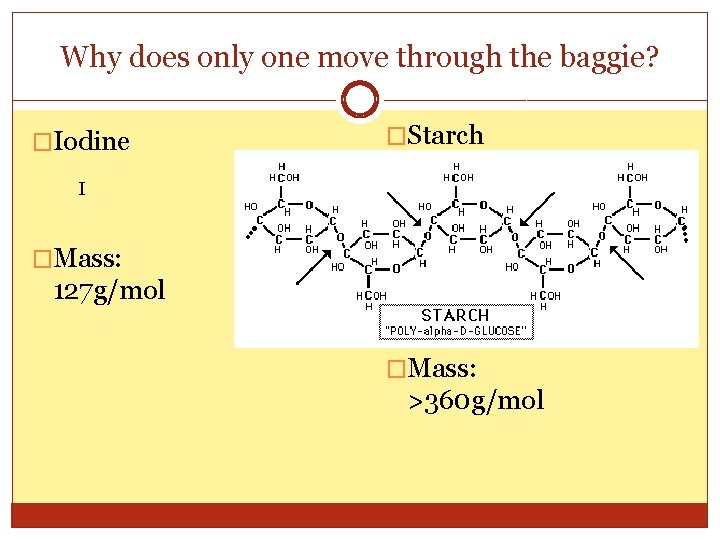
Why does only one move through the baggie? �Iodine �Starch I �Mass: 127 g/mol �Mass: >360 g/mol
- Slides: 13