Cell EMF Standard Reduction HalfCell Potentials Consider Zns




- Slides: 19

Cell EMF Standard Reduction (Half-Cell) Potentials • Consider Zn(s) Zn 2+(aq) + 2 e-. We measure Ecell relative to the SHE (cathode): E cell = E red(cathode) + E ox (anode) 0. 76 V = 0 V + E ox(anode). • Therefore, E ox(anode) = +0. 76 V. • Standard reduction potentials must be written as reduction reactions: Zn 2+(aq) + 2 e- Zn(s), E red = -0. 76 V.

• Since E red = -0. 76 V we conclude that the reduction of Zn 2+ in the presence of the SHE is not spontaneous. • The oxidation of Zn with the SHE is spontaneous. • Changing the stoichiometric coefficient does not affect E red. • Therefore, 2 Zn 2+(aq) + 4 e- 2 Zn(s), E red = -0. 76 V. • Reactions with E red > 0 are spontaneous reductions relative to the SHE.

• Reactions with E red < 0 are spontaneous oxidations relative to the SHE. • The larger the difference between E red values, the larger E cell. • In a voltaic (galvanic) cell (spontaneous) E red(cathode) is more positive than E red(anode). • Recall

Examples – Balance the following equations, and calculate E°cell for each: 1. Cr 3+ + Cl 2 (g) Cr 2 O 72 - + Cl- 2. Cu 2+ + Mg (s) Mg 2+ + Cu (s) 3. IO 3 - + Fe 2+ Fe 3+ + I 2 4. Zn (s) + Ag+ Zn 2+ + Ag

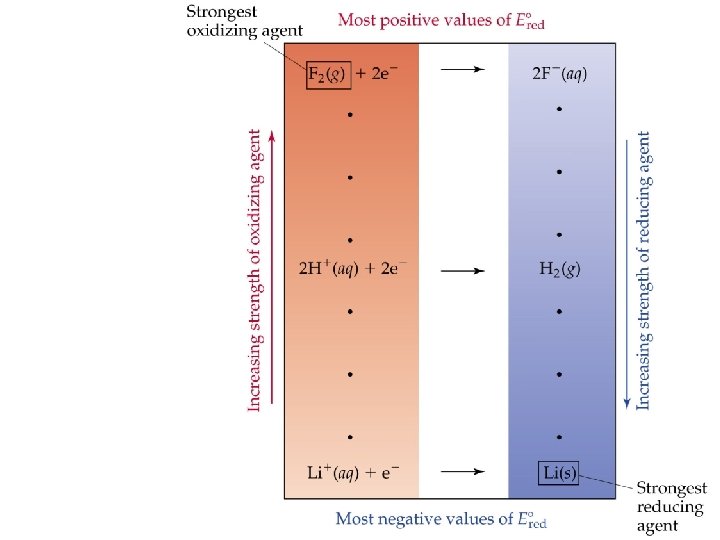
• • Oxidizing and Reducing Agents The more positive E red the stronger the oxidizing agent on the left. The more negative E red the stronger the reducing agent on the right. A species on the higher to the left of the table of standard reduction potentials will spontaneously oxidize a species that is lower to the right in the table. That is, F 2 will oxidize H 2 or Li; Ni 2+ will oxidize Al(s).


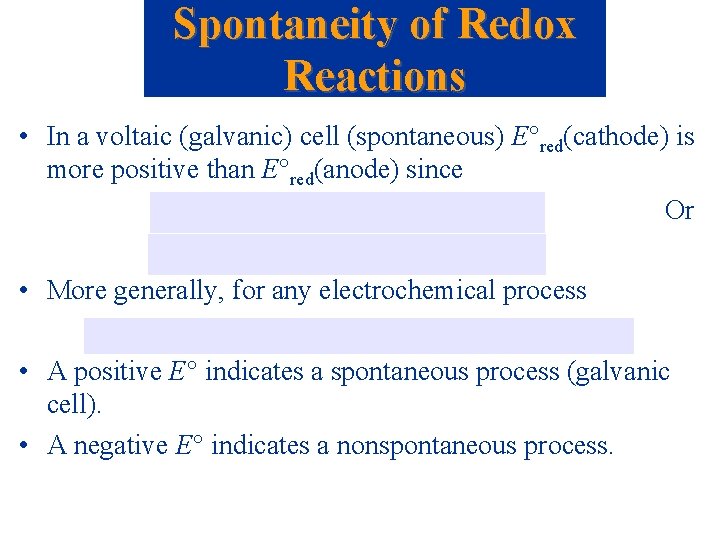
Spontaneity of Redox Reactions • In a voltaic (galvanic) cell (spontaneous) E red(cathode) is more positive than E red(anode) since Or • More generally, for any electrochemical process • A positive E indicates a spontaneous process (galvanic cell). • A negative E indicates a nonspontaneous process.

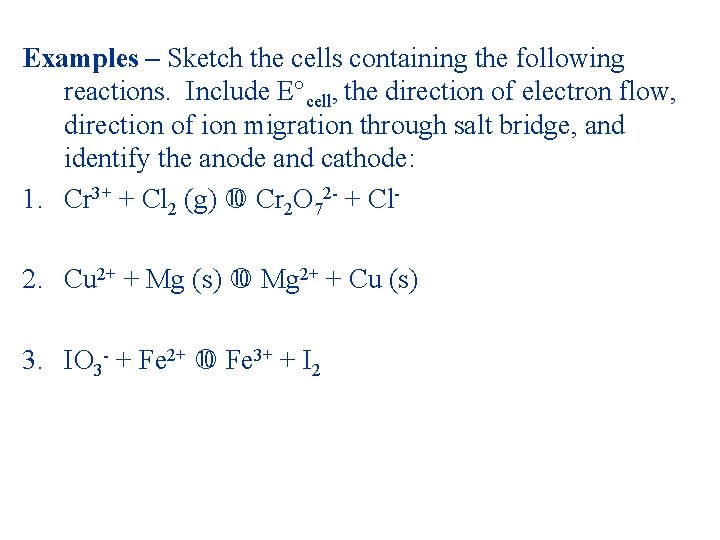
Examples – Sketch the cells containing the following reactions. Include E°cell, the direction of electron flow, direction of ion migration through salt bridge, and identify the anode and cathode: 1. Cr 3+ + Cl 2 (g) Cr 2 O 72 - + Cl- 2. Cu 2+ + Mg (s) Mg 2+ + Cu (s) 3. IO 3 - + Fe 2+ Fe 3+ + I 2

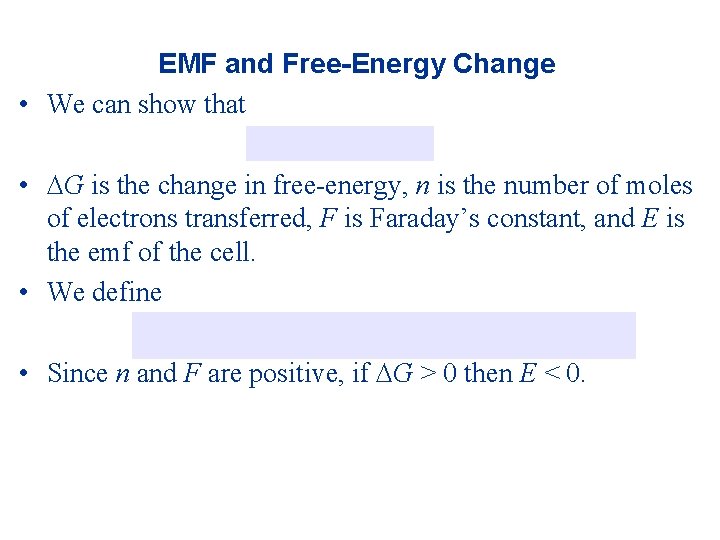
EMF and Free-Energy Change • We can show that • G is the change in free-energy, n is the number of moles of electrons transferred, F is Faraday’s constant, and E is the emf of the cell. • We define • Since n and F are positive, if G > 0 then E < 0.

Examples – Balance, and calculate E°cell and ΔG° for each reaction: 1. Mg (s) + Au 3+ Mg 2+ + Au (s) 2. Al (s) + Ag+ Al 3+ + Ag (s) 3. Cu 2+ + Na (s) Cu (s) + Na+

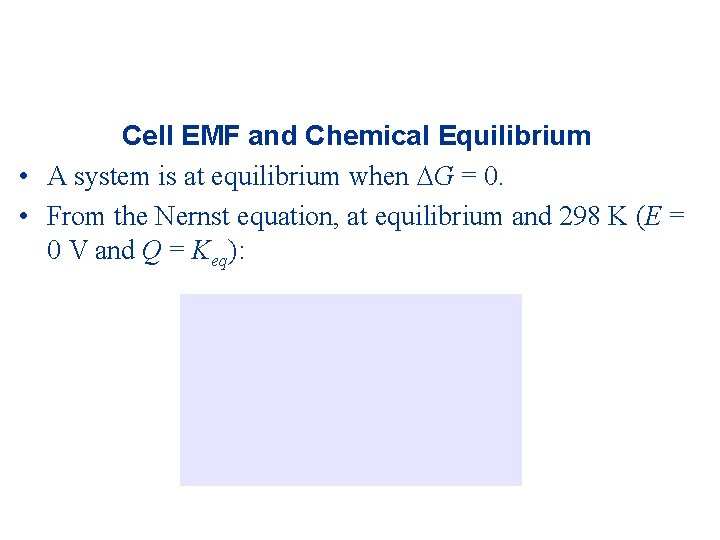
Cell EMF and Chemical Equilibrium • A system is at equilibrium when G = 0. • From the Nernst equation, at equilibrium and 298 K (E = 0 V and Q = Keq):

Batteries • A battery is a self-contained electrochemical power source with one or more voltaic cell. • When the cells are connected in series, greater emfs can be achieved.

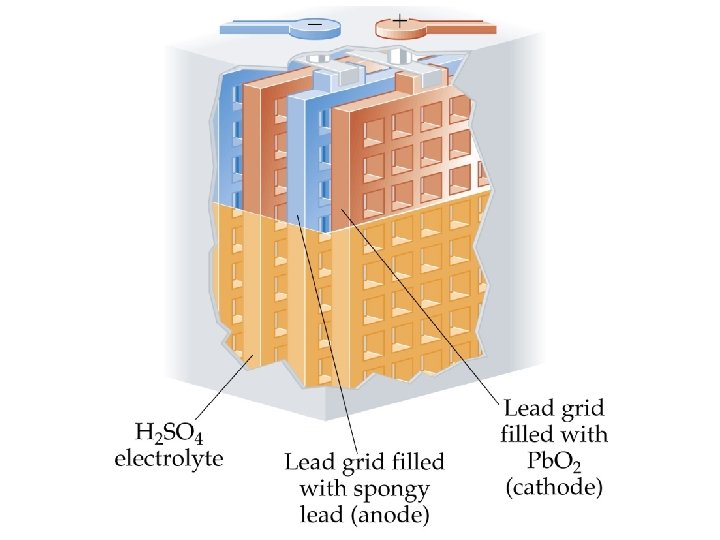
Lead-Acid Battery • A 12 V car battery consists of 6 cathode/anode pairs each producing 2 V. • Cathode: Pb. O 2 on a metal grid in sulfuric acid: Pb. O 2(s) + SO 42 -(aq) + 4 H+(aq) + 2 e- Pb. SO 4(s) + 2 H 2 O(l) • Anode: Pb(s) + SO 42 -(aq) Pb. SO 4(s) + 2 e-


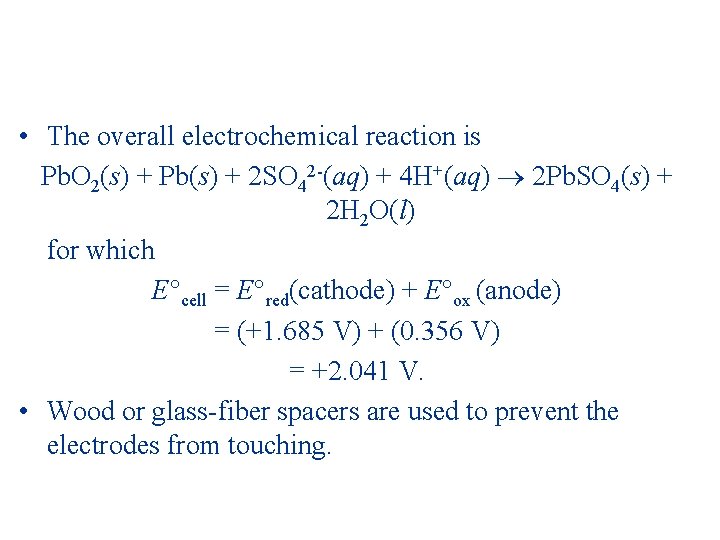
• The overall electrochemical reaction is Pb. O 2(s) + Pb(s) + 2 SO 42 -(aq) + 4 H+(aq) 2 Pb. SO 4(s) + 2 H 2 O(l) for which E cell = E red(cathode) + E ox (anode) = (+1. 685 V) + (0. 356 V) = +2. 041 V. • Wood or glass-fiber spacers are used to prevent the electrodes from touching.

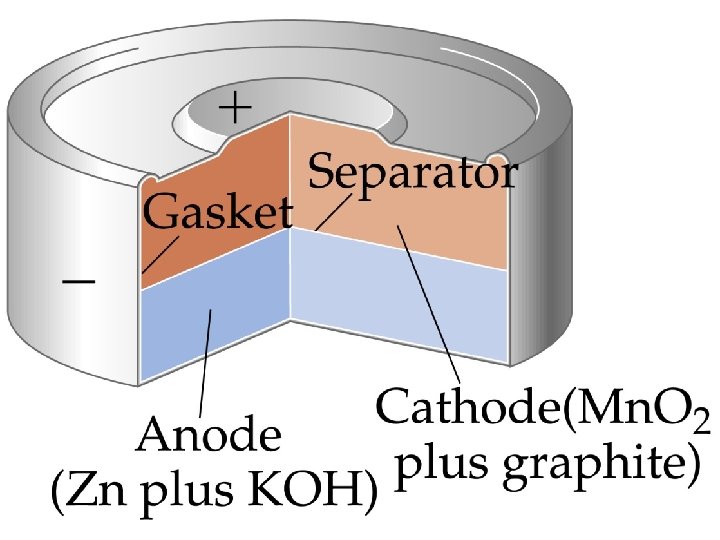
Alkaline Battery • Anode: Zn cap: Zn(s) Zn 2+(aq) + 2 e • Cathode: Mn. O 2, NH 4 Cl and C paste: 2 NH 4+(aq) + 2 Mn. O 2(s) + 2 e- Mn 2 O 3(s) + 2 NH 3(aq) + 2 H 2 O(l) • The graphite rod in the center is an inert cathode. • For an alkaline battery, NH 4 Cl is replaced with KOH.

• Anode: Zn powder mixed in a gel: Zn(s) Zn 2+(aq) + 2 e • Cathode: reduction of Mn. O 2.


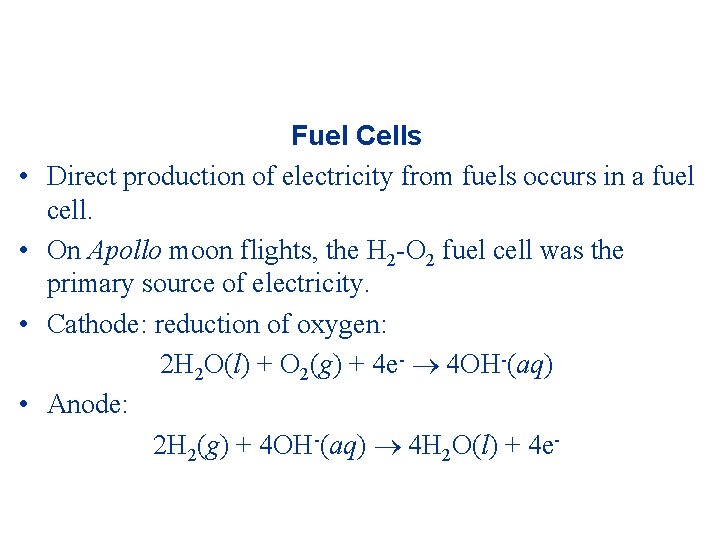
• • Fuel Cells Direct production of electricity from fuels occurs in a fuel cell. On Apollo moon flights, the H 2 -O 2 fuel cell was the primary source of electricity. Cathode: reduction of oxygen: 2 H 2 O(l) + O 2(g) + 4 e- 4 OH-(aq) Anode: 2 H 2(g) + 4 OH-(aq) 4 H 2 O(l) + 4 e-