CELL BIOLOGY TECHNIQUES Visualize cells Microscopy Organelles Fractionation
CELL BIOLOGY TECHNIQUES Visualize cells - Microscopy Organelles – Fractionation of subcellular components Culturing cells
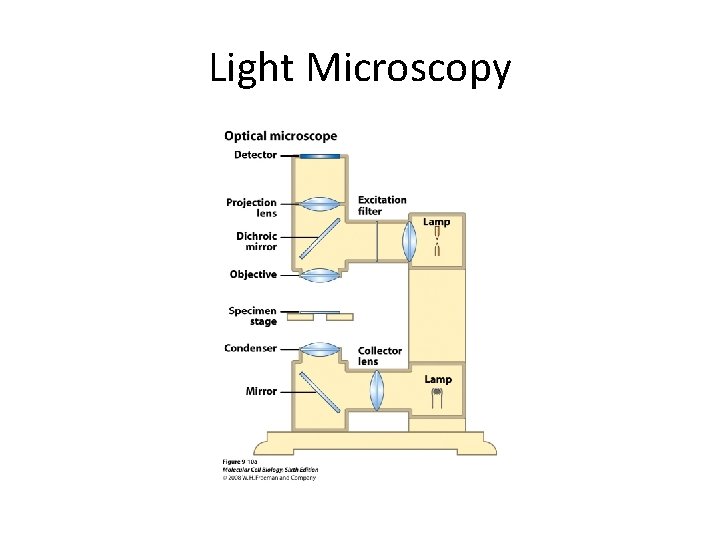
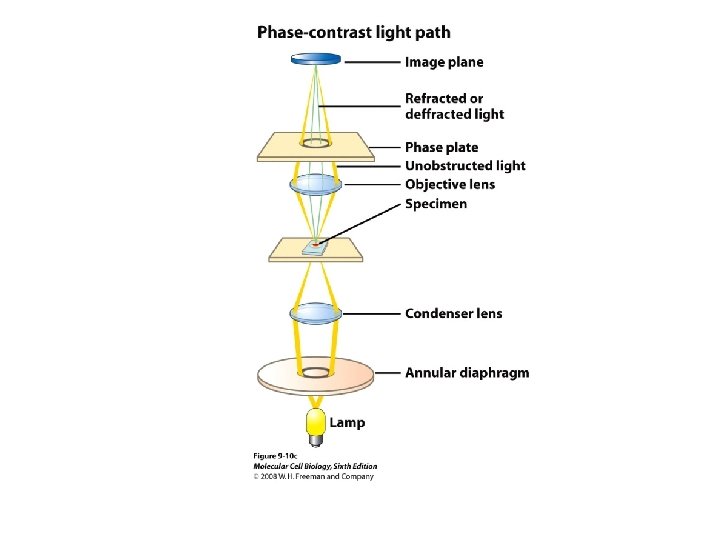
Light Microscopy

Light Microscopy • Resolution of 0. 2µm • Magnification – objective and projection lens • Resolution – D = 0. 61λ/N sin α Resolution is improved by using shorter wavelengths or increasing either N or α.
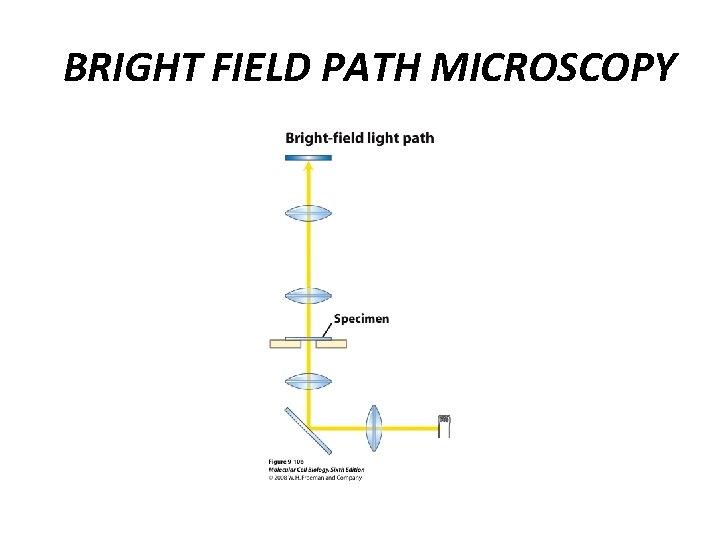
BRIGHT FIELD PATH MICROSCOPY
Visualize unstained living cells • Phase Contrast microscopy – Thin layers of cells but not thick tissues • Differential Interference contrast – Suited for extremely small details and thick objects – Thin optical section through the object
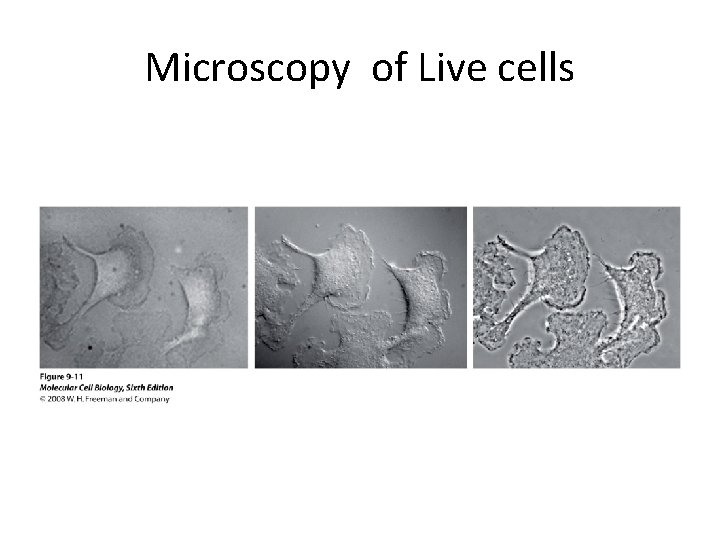
Microscopy of Live cells
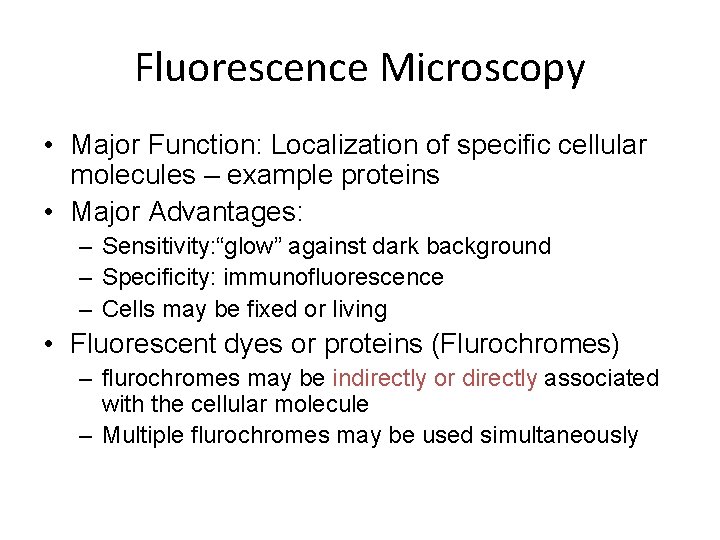
Fluorescence Microscopy • Major Function: Localization of specific cellular molecules – example proteins • Major Advantages: – Sensitivity: “glow” against dark background – Specificity: immunofluorescence – Cells may be fixed or living • Fluorescent dyes or proteins (Flurochromes) – flurochromes may be indirectly or directly associated with the cellular molecule – Multiple flurochromes may be used simultaneously
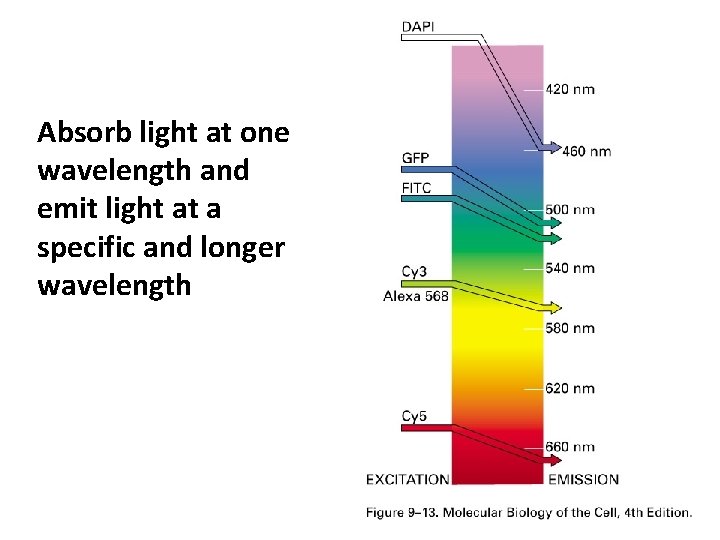
Absorb light at one wavelength and emit light at a specific and longer wavelength
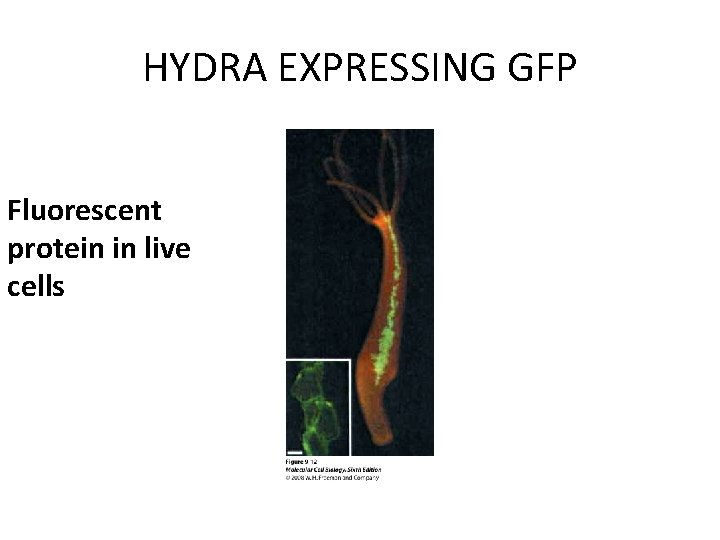
HYDRA EXPRESSING GFP Fluorescent protein in live cells
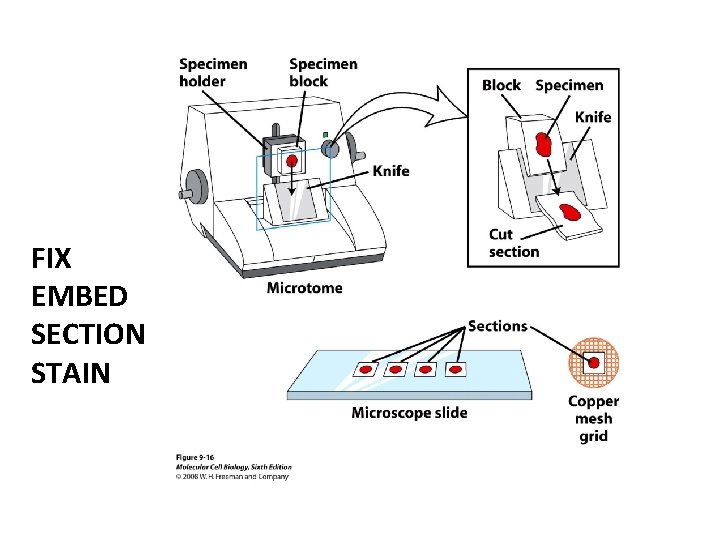
FIX EMBED SECTION STAIN
Immunofluorescence Microscopy and Specific Proteins • Fluorescently tagged primary anti body • Fluorescently tagged secondary antibody • Fluorescently labelled antibody to tagged proteins such as myc or FLAG
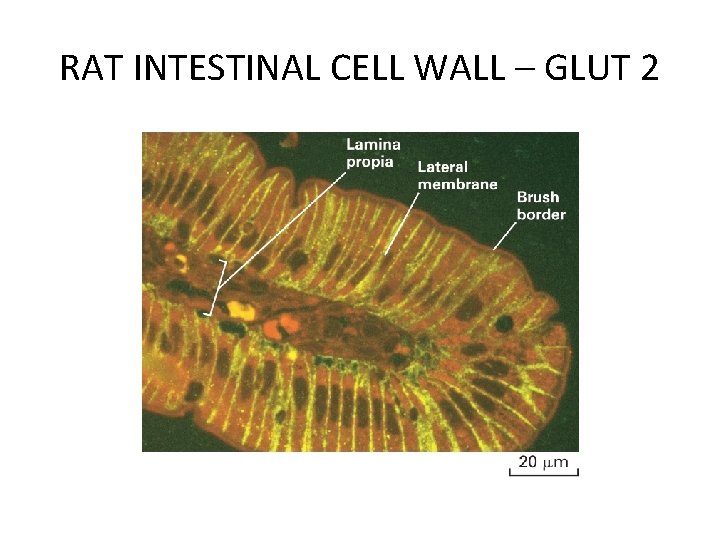
RAT INTESTINAL CELL WALL – GLUT 2
CONFOCAL AND DECONVOLUTION MICROSCOPY • This overcomes the limitations of Fluorescence microscopy – Blurrred images – Thick specimens
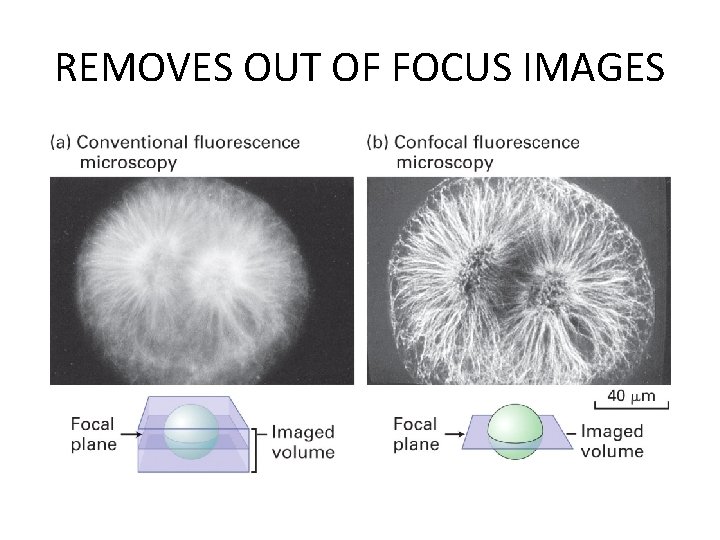
REMOVES OUT OF FOCUS IMAGES
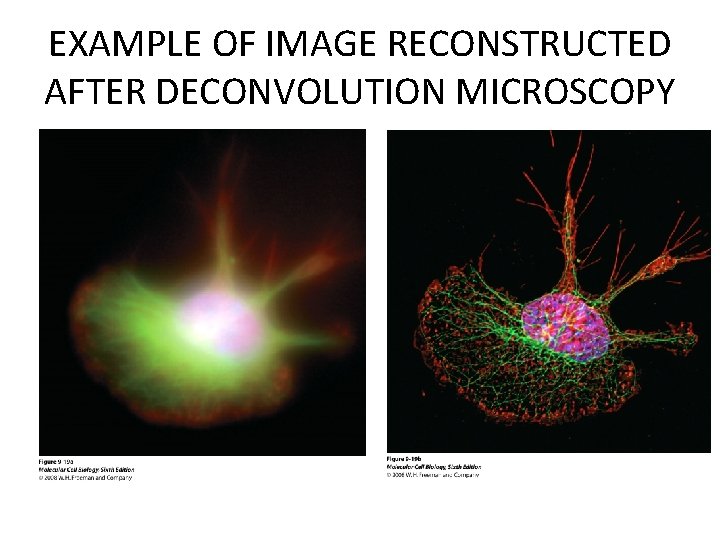
EXAMPLE OF IMAGE RECONSTRUCTED AFTER DECONVOLUTION MICROSCOPY
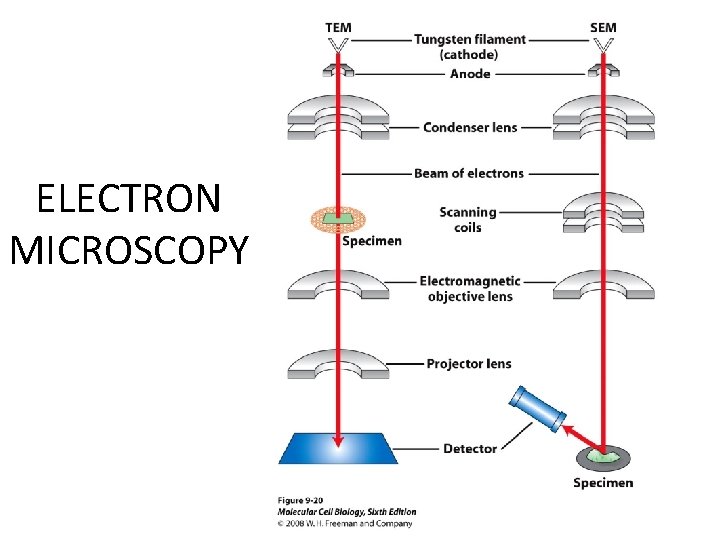
ELECTRON MICROSCOPY

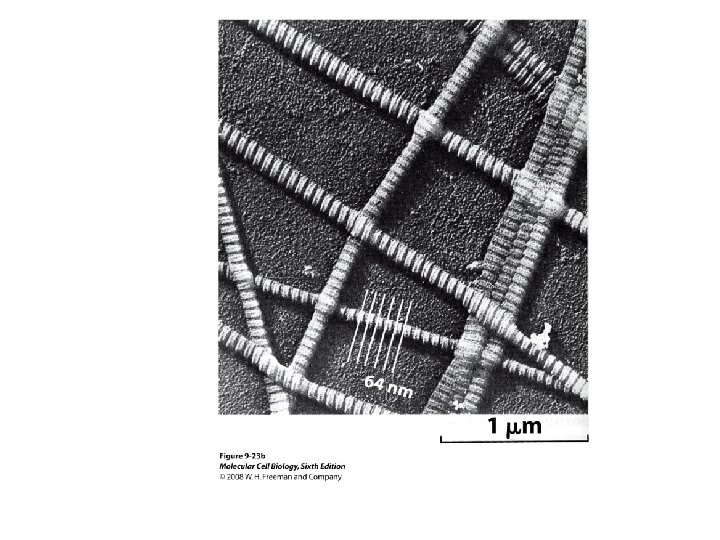
• Transmission EM – theoretically 0. 005 nm; practically 0. 1 nm – 1 nm (2000 x better than LM) – High – velocity electron beam passes through the sample – 50 -100 nm thick sections – 2 -D sectional image – surface details are revelaed – Subcellular organelles • Scanning EM – Resolution about 10 nm – Secondary electrons released from the metal coated unsectioned specimen – 3 -D surface image
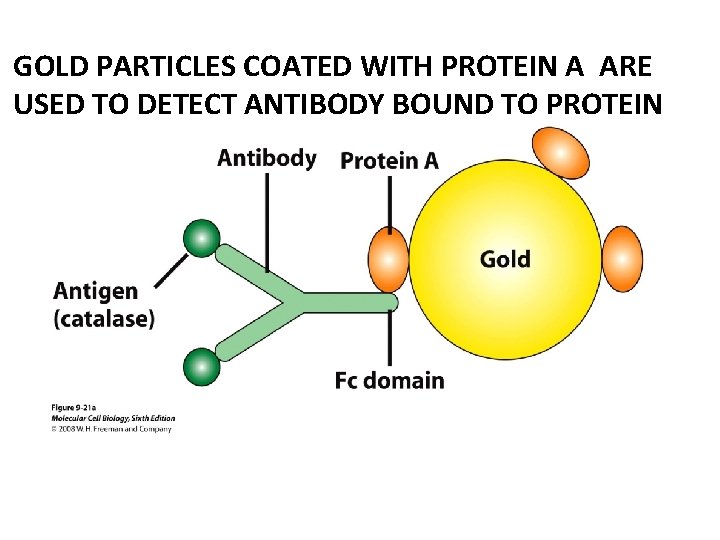
GOLD PARTICLES COATED WITH PROTEIN A ARE USED TO DETECT ANTIBODY BOUND TO PROTEIN
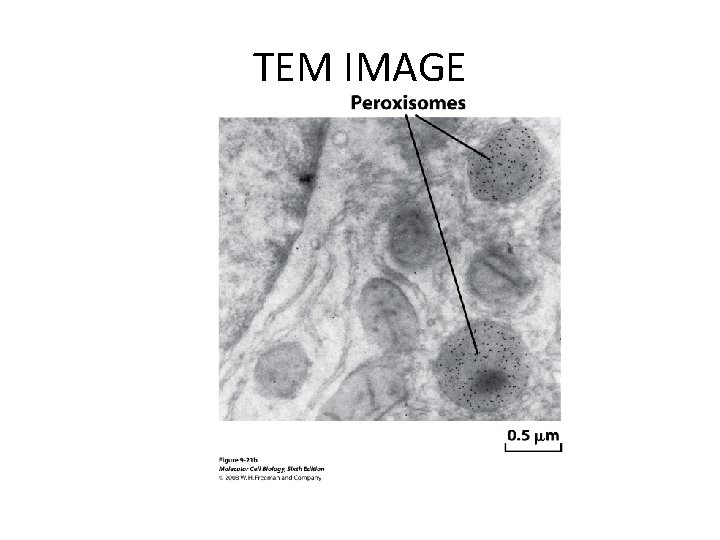
TEM IMAGE

CRYOELECTRON MICROSCOPY • HYDRATED, UNFIXED AND UNSTAINED SAMPLES • SAMPLES ARE OBSERVED IN ITS NATIVE HYDRATED STATE • METHOD - AN AQUEOUS SUSPENSION OF SAMPLE IS APLLIED ON A GRID AND HELP B Y A SPECIAL MOUNT • 5 nm RESOLUTION
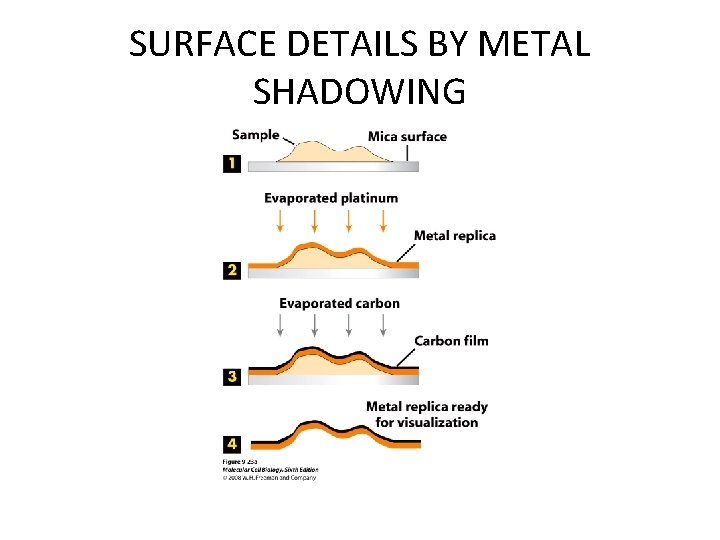
SURFACE DETAILS BY METAL SHADOWING
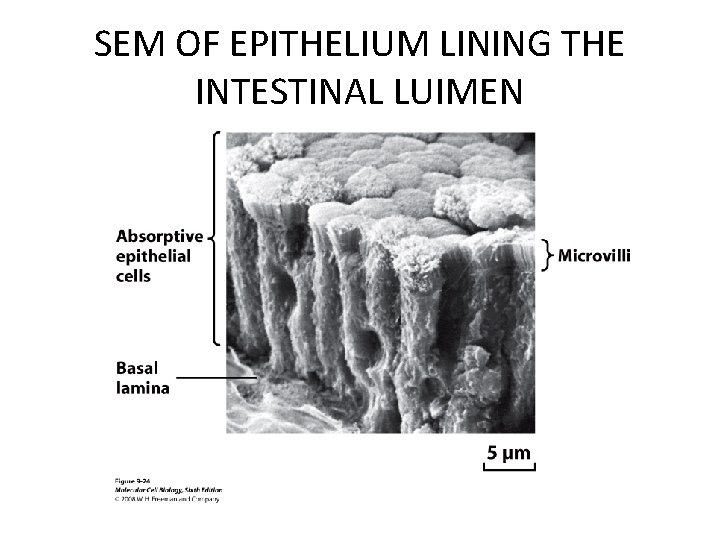
SEM OF EPITHELIUM LINING THE INTESTINAL LUIMEN
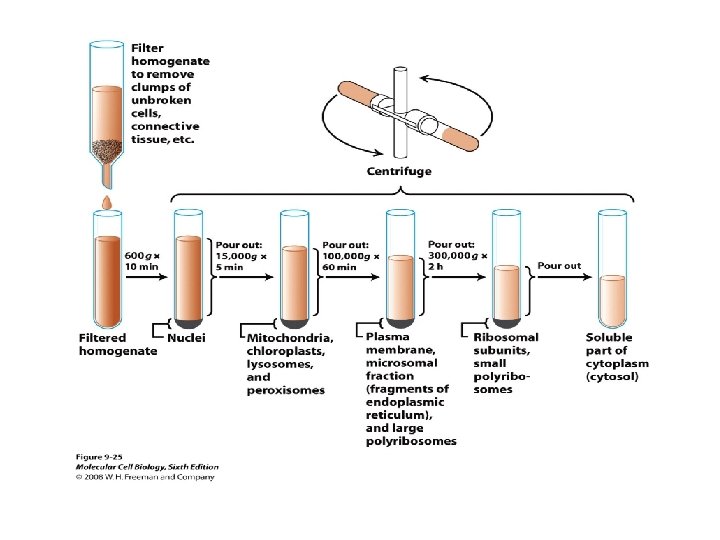
PURIFICATION OF CELL ORGANELLES • CELL DISRUPTION • SEPARATION OF DIFFERENT ORGANELLES USING CENTRIFUGATION • PREPARATION OF PURIFIED ORGANELLES USING SPECIFIC ANTIBODIES
BREAKING OPEN PLASMA MEMBRANES IN CELLS • • CELLS ARE SUSPENDED IN ISOTONIC SUCROSE SONICATION HOMOGENIZATION CELLS IN HYPOTONIC SOLUTION – RUPTURE OF CELL MEMBRANES
SEPERATING ORGANELLES • DIFFERENTIAL CENTRIFUGATION • DENSITY GRADIENT CENTRIFUGATION
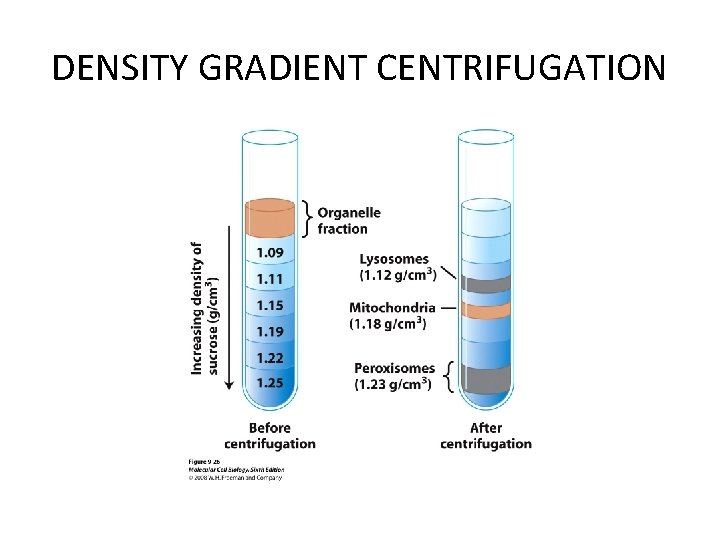
DENSITY GRADIENT CENTRIFUGATION
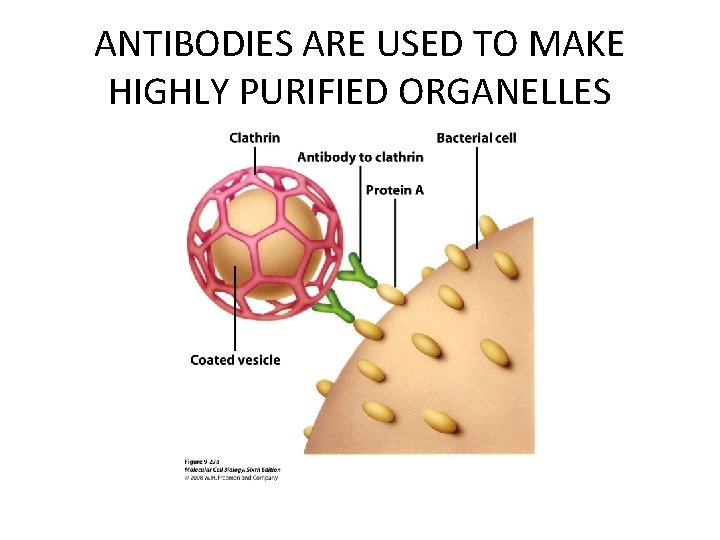
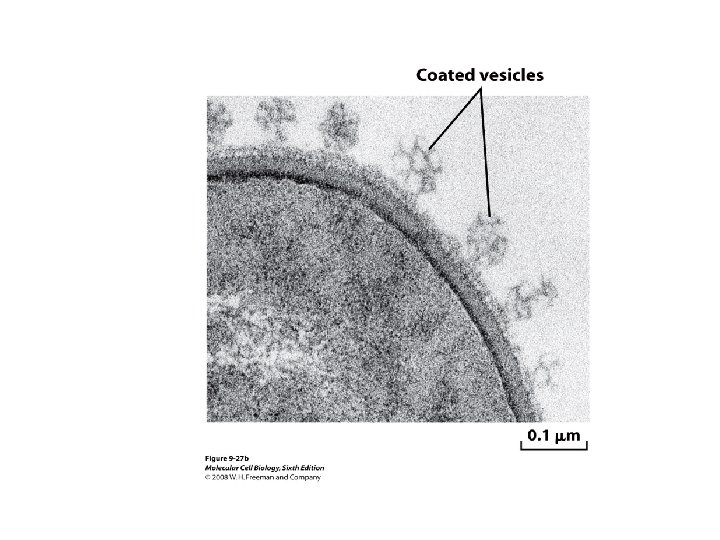
ANTIBODIES ARE USED TO MAKE HIGHLY PURIFIED ORGANELLES
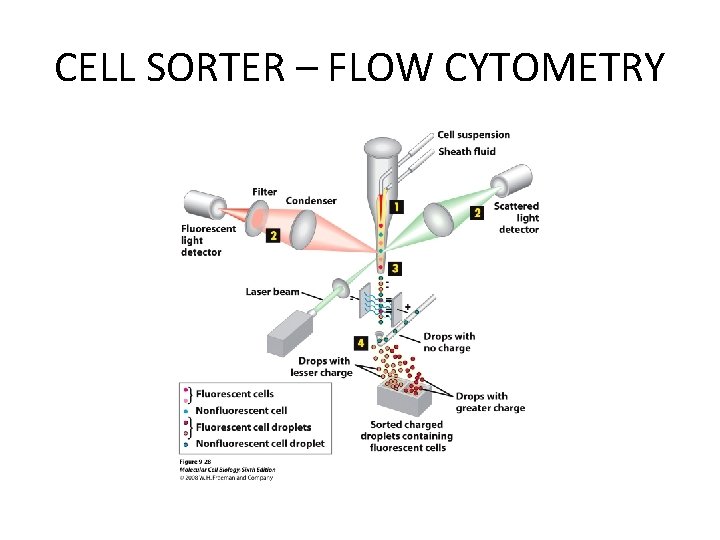
CELL SORTER – FLOW CYTOMETRY

CELL CULTURE REQUIREMENTS • SOLID MEDIA – Specially coated plastic dishes or flasks (CAMs’) – Agar as the medium GROWTH MEDIA Rich in nutrients- amino acids, vitamins, salts fatty acids, glucose, serum provides the different growth factors,
TYPES OF CULTURED CELLS • PRIMARY CELL CULTURES – DIFFERENTIATE IN CELL CULTURE • CELL STRAIN – ALSO HAVE A FINITE LIFE SPAN (FROM A PRIMARY CULTURE) • CELL LINE - INDEFINITE LIFE SPAN
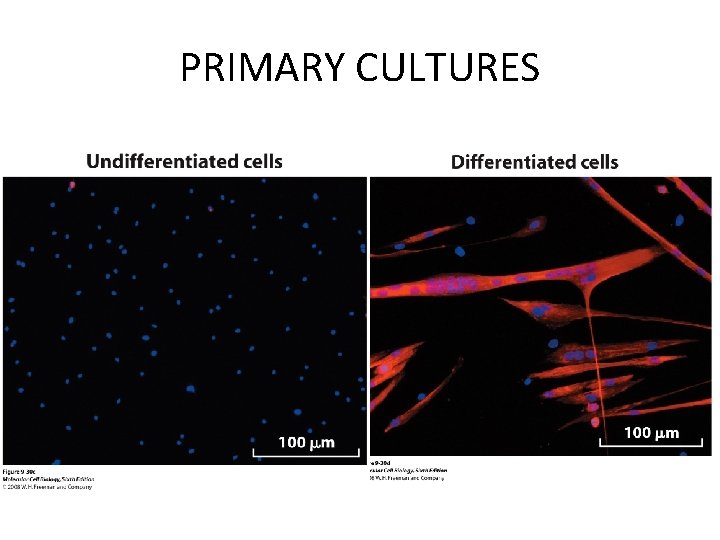
PRIMARY CULTURES
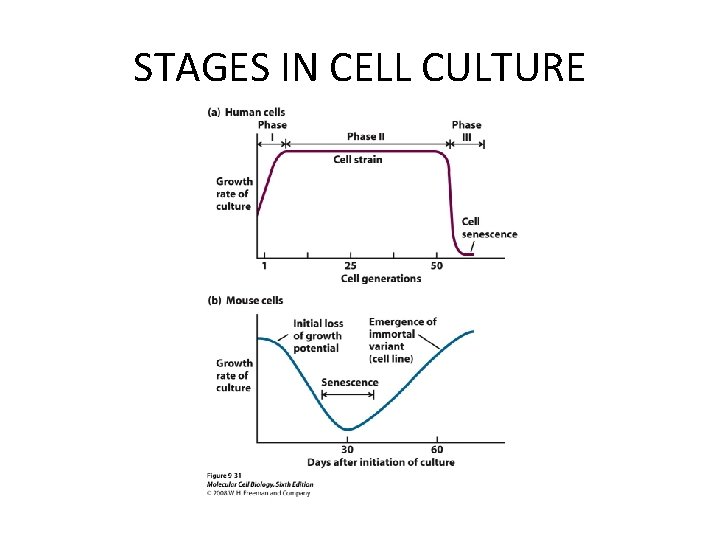
STAGES IN CELL CULTURE
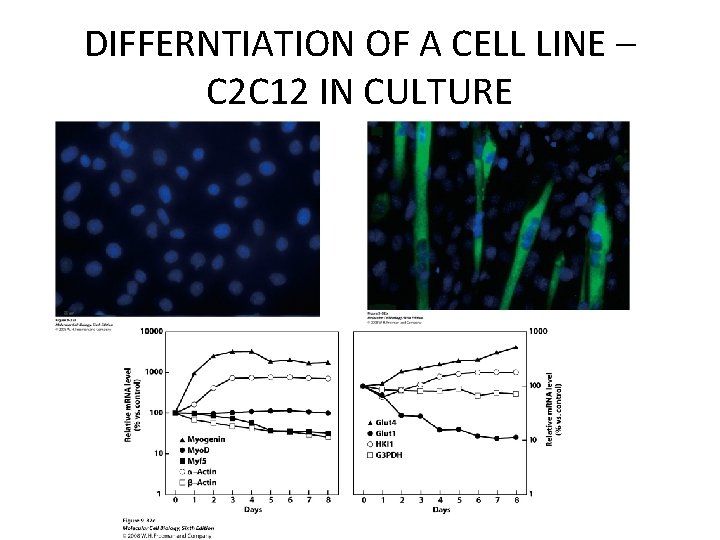
DIFFERNTIATION OF A CELL LINE – C 2 C 12 IN CULTURE

HOMEWORK-1 • CHAPTER 9 – REVIEW CONCEPTS QUESTIONS -2, 5, 7, 9 – ANALYZE THE DATA DUE NEXT WEEK IN THE WORKSHOPS
- Slides: 38