CDRHs Expedited Access Pathway Owen Faris Ph D















- Slides: 16

CDRH’s Expedited Access Pathway Owen Faris, Ph. D. Clinical Trials Director Office of Device Evaluation Center for Devices and Radiological Health

CDRH’s Vision Statement Patients in the U. S. have access to highquality, safe, and effective medical devices of public health importance first in the world.

Expedited Access Pathway (EAP) • Voluntary program for certain medical devices that demonstrate the potential to address unmet medical needs for life threatening or irreversibly debilitating diseases or conditions. • Goal: help patients have more timely access to these medical devices by expediting their development, assessment, and review, while preserving our statutory standards for safety and effectiveness and protecting patients

EAP Guidance • Final Guidance issued and implemented April 13, 2015 • Applies to PMA and De Novo devices

EAP Criteria 1. The device is intended to treat or diagnose a life-threatening or irreversibly debilitating disease or condition. 2. The device has the potential to address an unmet need. 3. The sponsor submits an acceptable draft Data Development Plan (DDP). – The DDP must be sufficient to support FDA’s review. However, it is not essential that FDA and the sponsor agree on the DDP at the time of EAP designation. 5

Benefits of EAP • More interaction with FDA during device development and IDE process • Senior CDRH management involvement • Case manager • Priority Review for PMA submissions • Potential to accept greater uncertainty for approval if available data demonstrate a reasonable assurance of safety and effectiveness • Collaborative creation of a “Data Development Plan” 6

Premarket-Postmarket Balance • Apply concepts of CDRH’s Benefit-Risk Guidance • Consider device’s potential to address critical unmet needs • FDA may accept less certainty regarding the benefit-risk profile of EAP devices subject to a PMA at the time of premarket approval as long as the data still support a reasonable assurance of safety and effectiveness. 7

Premarket-Postmarket Balance • Examples: – Intermediate endpoints – Surrogate endpoints – Two-phase studies 8

Data Development Plan • Data collection plan through marketing application and post-approval – Preclinical – Premarket clinical – Postmarket • A timeline for device development and pre and postmarket data collection 9

EAP Process • If EAP is granted, high priority interaction initiated to reach agreement on data development plan – Short timelines for FDA feedback and discussion – Division management involved in sponsor meetings – ODE and CDRH management informed of progress and involved as appropriate. • Continued commitment by FDA to be responsive and interactive as issues or changes arise 10

Current EAP Activity • • • 28 EAP designation requests received 14 granted 8 denied 4 under review 2 withdrawn (prior to EAP decision) 11

Lessons learned so far • Data Development Plans are challenging to work out – Some are very high level, some are very detailed depending on stage of development – Gaining agreement on the entire roadmap is new to both sponsors and FDA • But DDPs can help sponsor and FDA focus on key questions such as: – Whether additional testing is needed before clinical – The scope of premarket and post-market clinical data requirements 12

Lessons learned so far • There is a “sweet spot” for entry into EAP – Sponsors who apply for EAP very early in device development • May not be able to demonstrate potential to address unmet need • May not be able to develop long term data development plan – Sponsors who apply for EAP late in device evaluation miss out on some of the critical benefits – Sweet spot: Sponsors who have some encouraging data and are ready to think about the plan for getting to market 13

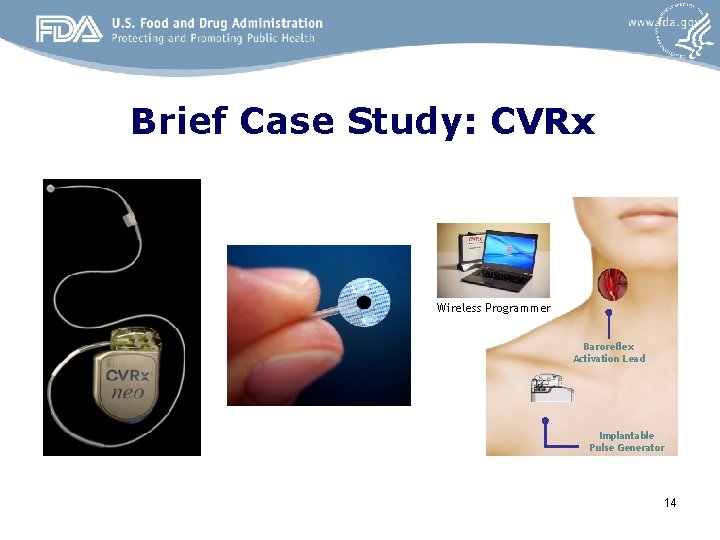
Brief Case Study: CVRx Wireless Programmer Baroreflex Activation Lead Implantable Pulse Generator 14

Company Experience (provided by CVRx) • Revised program in April 2015 ‒ Clearly written guidance document; many new practical clinical and process aspects ‒ Filed the EAP application request per guidance including rationale and justification of unmet clinical need in specific patient population and lack of alternate therapies along with a full development plan (May 2015) o ‒ • Received EAP designation within 30 days (June 2015) Engagement with FDA ‒ Pre-submission with protocol proposal (July 2015) ‒ In-person meeting within 5 weeks (August 2015) ‒ Interactive conference calls September & October 2015 (biweekly or as needed), resulting in trial design agreement and subsequent IDE Supplement approval (November 2015) o • Provided clinical evidence that the proposed therapy had potential to be safe and effective in targeted population FDA required new discussion materials to be submitted 7 days prior to a conference call Recommendations (Overall positive experience) ‒ New process for FDA and the company – learning on both sides, plan to review the process with FDA review team and previous points of agreement throughout the process ‒ Start as early as possible ‒ Most beneficial to get to an interactive process with weekly calls/meetings if possible ‒ Establish goals and summarize next steps for every meeting/call to keep the process moving

Conclusions • The Expedited Access Pathway is an extension of CDRH’s broader efforts to bring important innovative medical devices to US patients sooner. • High level of sponsor interest in the program • As we gain experience and receive feedback from sponsors and FDA staff, we expect the program to evolve and improve 16