CDISC Use of LOINC Codes 2015 04 22




- Slides: 6

CDISC Use of LOINC Codes 2015 -04 -22

LOINC Code Definition • Component – Analyte: Sodium, Ig. G, HIV-1 Antibody • Property – Defines the nature of the UOM – Examples: Mass Concentration (mg/d. L), Count, Time • Time – Time span of test: Point in Time, 24 Hour Clearance • System – Specimen type: Whole Blood, Serum, Urine • Scale – Nature of the result value: Qualitative, Ordinal, Quantitative • Method – Test Method: Ion Selective Electrode (ISE), Dip Stick, Methodless

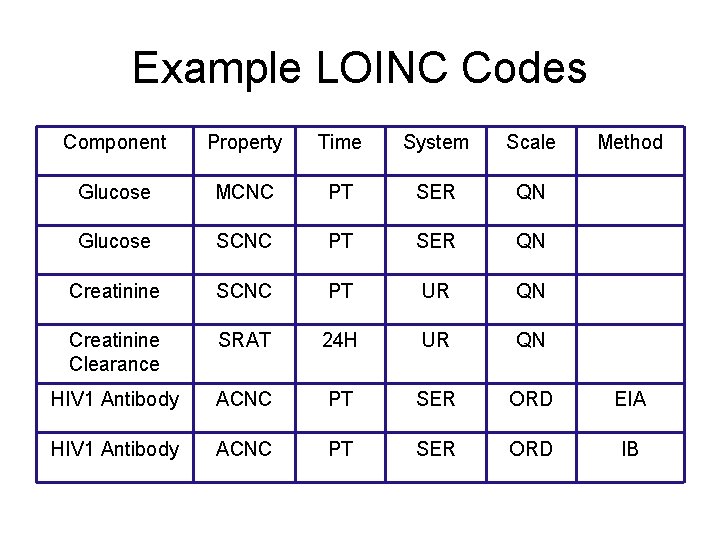
Example LOINC Codes Component Property Time System Scale Method Glucose MCNC PT SER QN Glucose SCNC PT SER QN Creatinine SCNC PT UR QN Creatinine Clearance SRAT 24 H UR QN HIV 1 Antibody ACNC PT SER ORD EIA HIV 1 Antibody ACNC PT SER ORD IB

CDISC Strengths • Post Coordinated Codes (CDISC) – Separate fields for each concept – Much better fit for Data Warehouses, Statistical Analysis, Informatics, Data Mining etc. • Pre-Coordinated Codes (LOINC) – Single Code includes multiple concepts – Much better fit for real time, machine to machine messaging

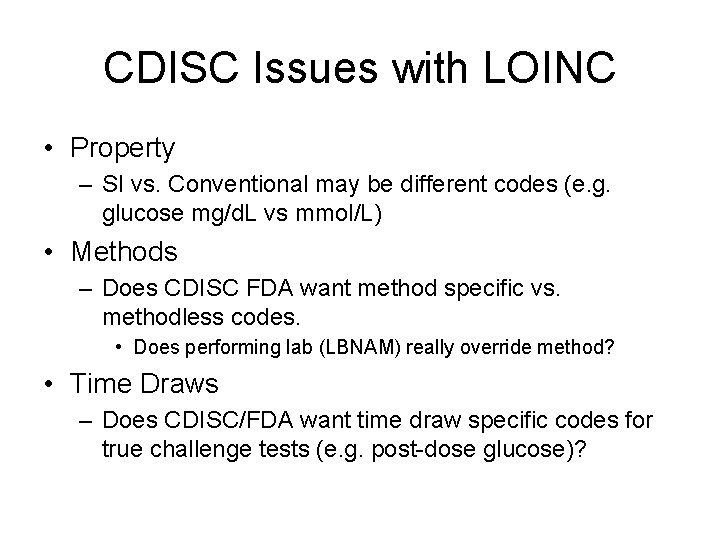
CDISC Issues with LOINC • Property – SI vs. Conventional may be different codes (e. g. glucose mg/d. L vs mmol/L) • Methods – Does CDISC FDA want method specific vs. methodless codes. • Does performing lab (LBNAM) really override method? • Time Draws – Does CDISC/FDA want time draw specific codes for true challenge tests (e. g. post-dose glucose)?

Why/How to Use LOINC • What is the business driver to use LOINC Codes? • Should LOINC codes be in the transmission model, or as metadata in SHARE? • If we do LOINC codes (US based), why not SNOMED (EU, Canada, Australia based)?