CDISC Implementation on a Rheumatoid Arthritis Project Partnership


















- Slides: 40

CDISC Implementation on a Rheumatoid Arthritis Project Partnership Patricia Gerend, Olivier Leconte, Chris Price, Michelle Zhang Genentech, Inc. and Roche Products Limited September 2009 Genentech Inc. Confidential

CDISC Background q CDISC: Clinical Data Interchange Standards Consortium q Founded around 1997 q Started by biotech / pharma staff 4 Common standards would make sponsors more efficient 4 Common standards would simplify FDA reviewers’ jobs q Used nationally, somewhat internationally q Used by industry, academic, coop, and regulatory groups 4 Common standards would accommodate cross-company, crossmolecule monitoring q Many CDISC branches 4 SDTM (Submission Data Tabulation Model – raw data) 4 ADa. M (Analysis Data Model – derived data) 4 Others for protocols, information exchange, lab data, CRF data, etc. 2 Genentech Inc. Confidential

Project Background q Pharma / Biotech Collaboration: Roche and Genentech q Rheumatoid Arthritis new molecule q Several new clinical studies getting started q Decision to do all work on Roche system q Different proprietary data standards at each company q New industry standard of CDISC q Neither company had production/filing CDISC experience q Genentech had performed 2 pilot CDISC projects, one with Meta. Xceed, another with Pharma. Stat, where vendors did modeling and programming 3 Genentech Inc. Confidential

CDISC: To Use or Not to Use q Decision to use CDISC 11/2007 4 Could be required by FDA at submission time 4 Avoids time and hassle of dealing with each other’s proprietary data standards 4 Provides growth opportunity for staff 4 Opportune timing since project just getting started 4 Quick management buy-in 4 Genentech Inc. Confidential

Tasks Required for CDISC Implementation q Intelligence gathering q Documenting standards q SDTM 4 Modeling of CRFs 4 Controlled Terminology 4 Conversion Specifications 4 Conversions q ADa. M 4 Analysis Database Design 4 Metadata and Specifications Structures 4 Derivations q Electronic submission to FDA 5 Genentech Inc. Confidential

Intelligence Gathering q Formal training: f 2 f, on-line (see CDISC web site) q Attendance at Bay Area CDISC Implementation Forum 4 Occurs approximately quarterly 4 Many SF bay area bio-pharm companies represented 4 CDISC organization speakers 4 Cross-pollination of ideas/approaches q Discussions w/ internal staff versed in CDISC q Reading CDISC guides (yes, including the 299 -page SDTM -Implementation Guide [IG]!) 4 Well-organized 4 Comprehensive 4 Good examples 6 Genentech Inc. Confidential

SDTM Modeling Controlled Terminology Documentation Conversion Specifications Conversions Genentech Inc. Confidential

SDTM Modeling q Pick a version of the CDISC SDTM Implementation Guide (IG): v 3. 1. 2 q Pick a version of the CDISC Controlled Terminology (CT): Recent version issued before first database lock: 7 July 2009 4 Note: No link between IG and CT q Define naming conventions for user-defined data domains 4 X_ for Interventions (example, XP for Previous Procedures) 4 Y_ for Events (example, YI for Previous Immunizations) 4 Z_ for Findings (example, ZJ for Tender and Swollen Joint Counts) q Define standard ways to handle non-standard data, such as “Other, specify” q Document conventions, modeling decisions, changes, project-specific controlled terminology 8 Genentech Inc. Confidential

SDTM Modeling Documentation q Value of documentation, though sometimes tedious, cannot be overstated q Document name: SDTM Modeling Information q Document sections: 4 Conventions for SDTM Modeling 4 CRF -> SDTM Domain Map 4 SDTM Domain -> CRF Map 4 Changes to Annotations since First Draft 9 Genentech Inc. Confidential

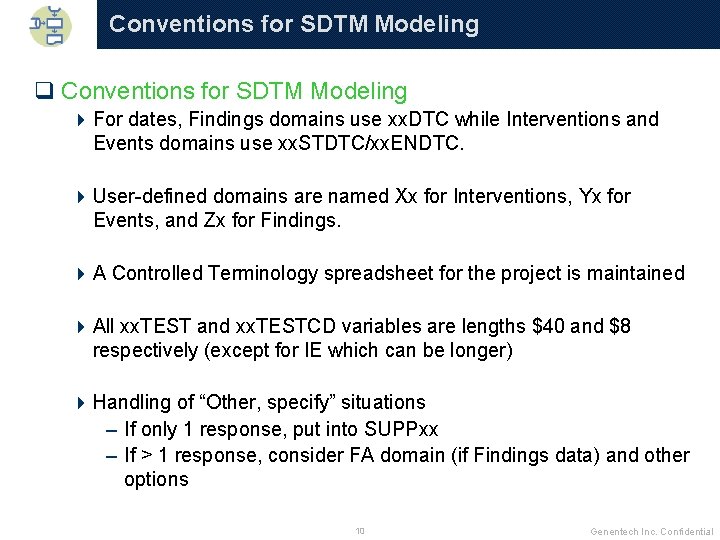
Conventions for SDTM Modeling q Conventions for SDTM Modeling 4 For dates, Findings domains use xx. DTC while Interventions and Events domains use xx. STDTC/xx. ENDTC. 4 User-defined domains are named Xx for Interventions, Yx for Events, and Zx for Findings. 4 A Controlled Terminology spreadsheet for the project is maintained 4 All xx. TEST and xx. TESTCD variables are lengths $40 and $8 respectively (except for IE which can be longer) 4 Handling of “Other, specify” situations – If only 1 response, put into SUPPxx – If > 1 response, consider FA domain (if Findings data) and other options 10 Genentech Inc. Confidential

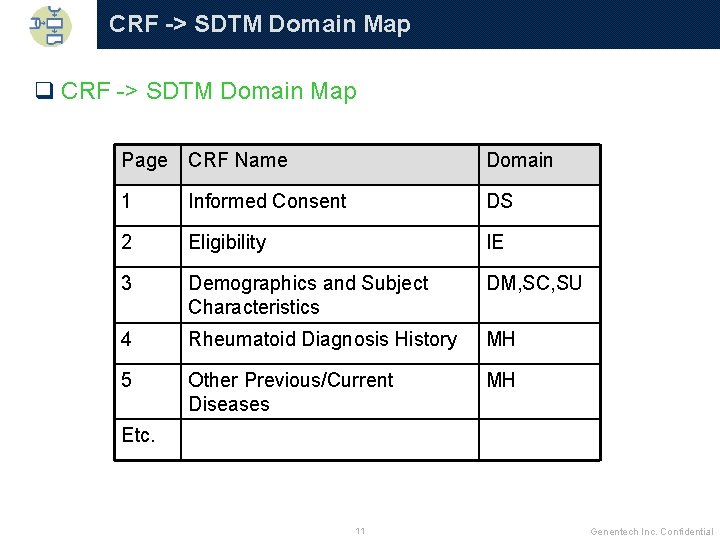
CRF -> SDTM Domain Map q CRF -> SDTM Domain Map Page CRF Name Domain 1 Informed Consent DS 2 Eligibility IE 3 Demographics and Subject Characteristics DM, SC, SU 4 Rheumatoid Diagnosis History MH 5 Other Previous/Current Diseases MH Etc. 11 Genentech Inc. Confidential

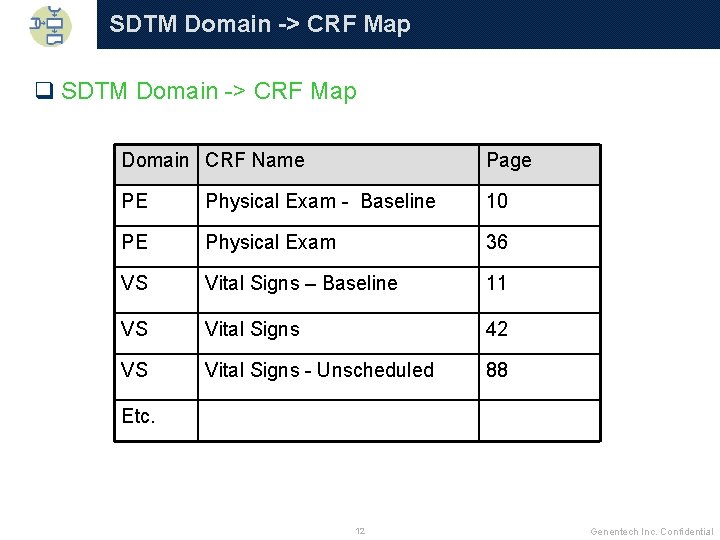
SDTM Domain -> CRF Map q SDTM Domain -> CRF Map Domain CRF Name Page PE Physical Exam - Baseline 10 PE Physical Exam 36 VS Vital Signs – Baseline 11 VS Vital Signs 42 VS Vital Signs - Unscheduled 88 Etc. 12 Genentech Inc. Confidential

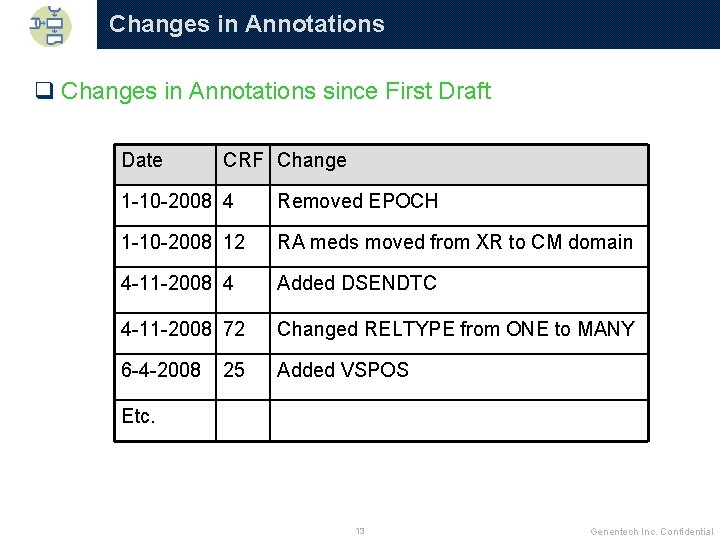
Changes in Annotations q Changes in Annotations since First Draft Date CRF Change 1 -10 -2008 4 Removed EPOCH 1 -10 -2008 12 RA meds moved from XR to CM domain 4 -11 -2008 4 Added DSENDTC 4 -11 -2008 72 Changed RELTYPE from ONE to MANY 6 -4 -2008 Added VSPOS 25 Etc. 13 Genentech Inc. Confidential

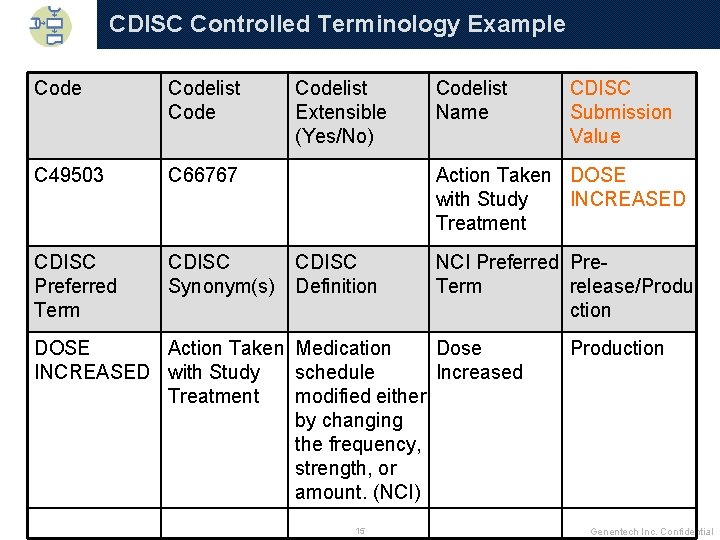
Controlled Terminology q Two Controlled Terminology (CT) documents: 4 CDISC organization 4 Project q Identify which version from CDISC organization to use across project q Identify and document terms specific to project to maintain consistency across studies q Map project values to CDISC CT where they exist q Put original values into --ORRES or SUPPQUAL if they differ substantially from CT values q Remember to check if a CDISC CT value list is extensible q Identify a Clinical Scientist to use for input into mappings from original to CT values 14 Genentech Inc. Confidential

CDISC Controlled Terminology Example Codelist Code C 49503 C 66767 CDISC Preferred Term CDISC Synonym(s) Codelist Extensible (Yes/No) Codelist Name CDISC Submission Value Action Taken DOSE with Study INCREASED Treatment CDISC Definition NCI Preferred Pre. Term release/Produ ction DOSE Action Taken Medication Dose INCREASED with Study schedule Increased Treatment modified either by changing the frequency, strength, or amount. (NCI) 15 Production Genentech Inc. Confidential

CDISC Controlled Terminology q Covers many SDTM variable values q Is updated often, much more so than data models q Generally new rows are added as opposed to changing existing information q Is fairly long (over 1, 000 rows in the 7 July 2009 version) 16 Genentech Inc. Confidential

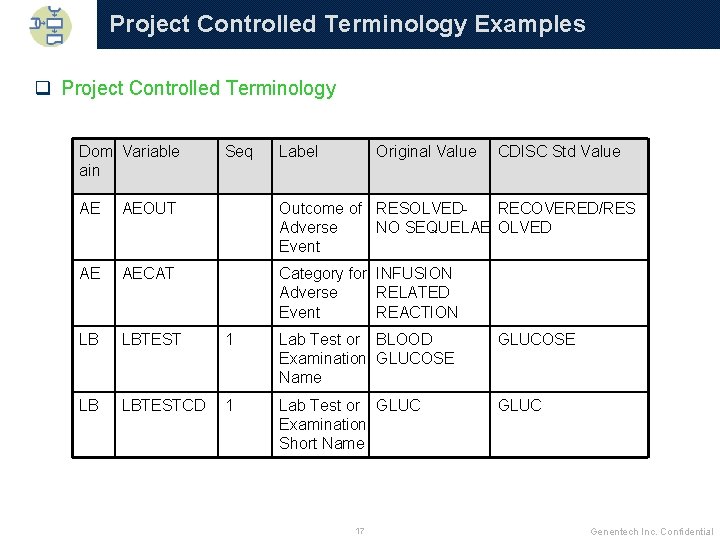
Project Controlled Terminology Examples q Project Controlled Terminology Dom Variable ain Seq Label Original Value CDISC Std Value AE AEOUT Outcome of RESOLVEDRECOVERED/RES Adverse NO SEQUELAE OLVED Event AE AECAT Category for INFUSION Adverse RELATED Event REACTION LB LBTEST 1 Lab Test or BLOOD Examination GLUCOSE Name GLUCOSE LB LBTESTCD 1 Lab Test or GLUC Examination Short Name GLUC 17 Genentech Inc. Confidential

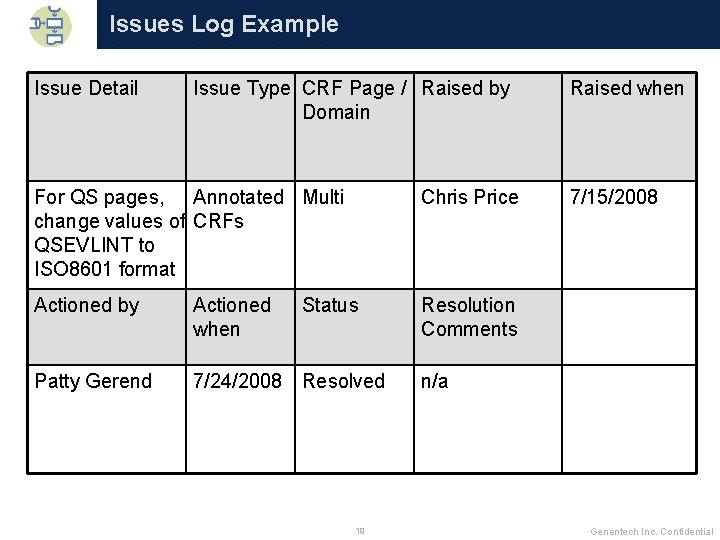
Issues Log q On a large team, it is easy to lose track of issues when addressed via email q Create an Issues Log 4 Put where accessible by whole team 4 Include columns indicating problem, who needed to solve it, and resolution 4 Refer to it often when making decisions to ensure consistency in project 18 Genentech Inc. Confidential

Issues Log Example Issue Detail Issue Type CRF Page / Raised by Domain For QS pages, Annotated Multi change values of CRFs QSEVLINT to ISO 8601 format Chris Price Actioned by Actioned when Resolution Comments Patty Gerend 7/24/2008 Resolved Status 19 Raised when 7/15/2008 n/a Genentech Inc. Confidential

SDTM Conversion Specifications q While many conversions are not difficult (e. g. , variable renames), some are, so documentation is helpful q Set up spreadsheet containing list of all possible variables in the domain and algorithms for populating them 20 Genentech Inc. Confidential

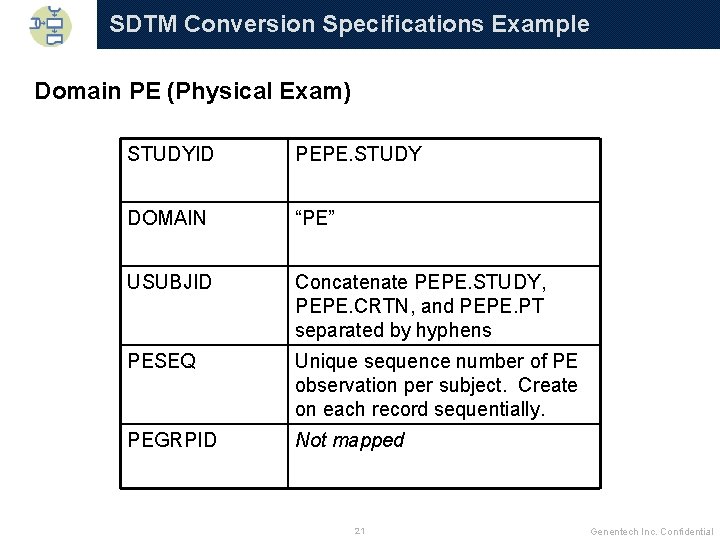
SDTM Conversion Specifications Example Domain PE (Physical Exam) STUDYID PEPE. STUDY DOMAIN “PE” USUBJID Concatenate PEPE. STUDY, PEPE. CRTN, and PEPE. PT separated by hyphens PESEQ Unique sequence number of PE observation per subject. Create on each record sequentially. PEGRPID Not mapped 21 Genentech Inc. Confidential

SDTM Conversion SAS Programs q Base SAS was used to perform the conversions from Oracle Clinical extract data to SDTM q Advantages over GUI tool used by non-team members 4 Project programmers can see entire picture of data derivations 4 Project programmers can participate in conversions 4 All data conversions/derivations are in one programming language with programs residing in one location to facilitate audit trail 22 Genentech Inc. Confidential

ADa. M Analysis Database Design Metadata and Specifications Structures Derivations Genentech Inc. Confidential

ADa. M Challenges q Metadata documentation q Vertical structures q LOCF (last observation carried forward) derivations q Analysis flags q Addition of rows versus columns 24 Genentech Inc. Confidential

ADa. M Metadata Documentation q Derivation text guidelines q Specifications structure decisions 25 Genentech Inc. Confidential

ADa. M Derivations Text Guidelines Examples q Text should be specific and detailed enough to allow recreation of the derived variable by the reader. q References to source variable names from a dataset other than the one being described should be two-level; e. g. , DM. RACE. If the source variable is from the same dataset as that being described, a one-level name is used; e. g. , RACE. q Use common English descriptions of operators and other symbols rather than using computer terms or math symbols; e. g. , "is missing" rather than "=. ". 26 Genentech Inc. Confidential

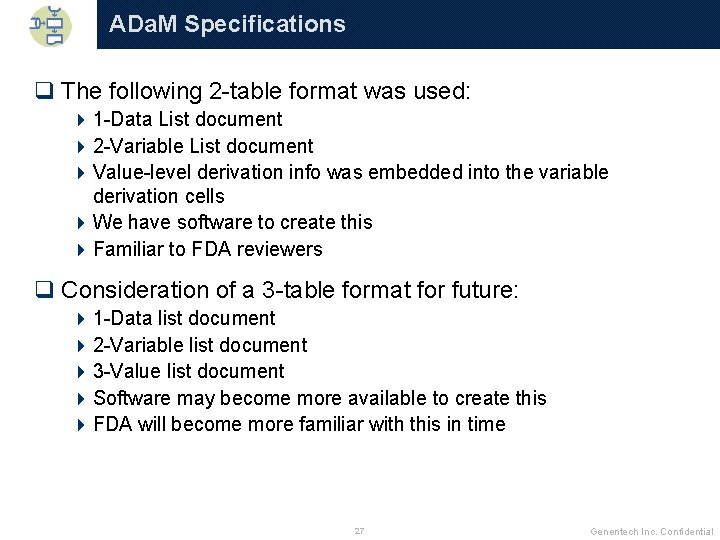
ADa. M Specifications q The following 2 -table format was used: 4 1 -Data List document 4 2 -Variable List document 4 Value-level derivation info was embedded into the variable derivation cells 4 We have software to create this 4 Familiar to FDA reviewers q Consideration of a 3 -table format for future: 4 1 -Data list document 4 2 -Variable list document 4 3 -Value list document 4 Software may become more available to create this 4 FDA will become more familiar with this in time 27 Genentech Inc. Confidential

ADa. M Metadata Columns q Dataset Metadata 4 Name 4 Description 4 Location 4 Structure 4 Purpose 4 Key Variables 4 Documentation (e. g. , Stat Plan, Reviewers’ Guide) q Variable Metadata 4 Name 4 Label 4 Type 4 Controlled Terms or Formats 4 Source or Derivation Method 28 Genentech Inc. Confidential

ADa. M Dataset Structures q Dataset structures 4 ADa. M structures are vertical 4 Genentech has standard SAS software designed to create and report horizontal analysis data 4 Roche has standard SAS software designed to create and report vertical analysis data q Decision to use Roche software on Roche system 29 Genentech Inc. Confidential

ADa. M Derivations Example q Last Observation Carried Forward (LOCF) 4 Always complicated regardless of data structure 4 Used ADa. M AVAL (analysis variable) and DTYPE (derivation type) variables together to identify observed and LOCF’ed values 4 In non-CDISC horizontal structures, only 1 variable was needed (it was called LOCF) 30 Genentech Inc. Confidential

ADa. M Analysis Flags q Many different ways of implementing ADa. M model q Had to decide between creating analysis flags for all reasonable analyses or for just those pre-specified: created all that seemed reasonable q Example analysis flag: ANL 1 FL indicates LOCF, excluding rescue and withdrawal q Decided to have ANLx. FL represent same concept across all ADa. M datasets, even though this means the value of x is not necessarily sequential in each dataset 4 Example: ADDS 1 contains ANL 1 FL, ANL 2 FL, ANL 3 FL, ANL 4 FL; ADDS 2 contains ANL 1 FL and ANL 4 FL q ADa. M model may still be evolving to handle more cases 31 Genentech Inc. Confidential

ADa. M Addition of Rows Versus Columns q Added a new column for a parameter-invariant functions of AVAL (analysis value) or BASE (baseline value) on the same row 4 “Parameter-invariant” means the function does not change from parameter to parameter and the meaning of the function is the same on all rows 4 Example: Change from Baseline q Added a new row for functions that involve more than one parameter or that require a new parameter 4 Example: Total number of tender joints is derived from each individual joint score, so total number is a new parameter and a new row 4 Example: LOCF imputation of missing values is put into a new row 32 Genentech Inc. Confidential

E-Sub SDTM ADa. M Genentech Inc. Confidential

Electronic Submission to FDA: SDTM q Followed SDTM-IGv 3. 1. 2 to the best of our abilities q Must still have SDTM structure validated q Plan to use Phase Forward’s Web. SDM product 4 Evaluation of SDTM structure adherence 4 Production of define. xml q Will also generate define. pdf to accommodate reviewers q Will submit dataset list, variable list, and controlled terminology q Expectation for SDTM data to load into FDA’s Janus data warehouse for cross-company, cross-drug monitoring 34 Genentech Inc. Confidential

Electronic Submission to FDA: ADa. M q Define. pdf, but not define. xml, will be generated and submitted q Define. xml production is time-consuming, costly, and problematic q Will submit dataset list and variable list q Not currently necessary for ADa. M data to be in FDA’s Janus data warehouse q ADa. M structure less stable than SDTM and could change later 35 Genentech Inc. Confidential

Efficiencies Gained q SDTM 4 First study took 8 months elapsed time 4 Second study took 3 months elapsed time 4 Third study took < 1 month elapsed time q ADa. M 4 First study took 4 months elapsed time 4 Second study took 2 months elapsed time 4 Third study took 1 month elapsed time q CDISC Overall 4 No haggling over each company’s proprietary data structures, so 6 months were saved here 36 Genentech Inc. Confidential

Conclusion q Results of decision to use CDISC with 2 companies not familiar with its structures 4 Successful SDTM conversion of 4 studies 4 Successful ADa. M derivation on 3 studies, so far 4 Intense CDISC learning across both companies 4 Information to move forward with organization-wide CDISC strategies q Successes yet to come 4 Electronic submission deliverables compilation 4 FDA evaluation of our efforts 4 Drug and indication approval!? 37 Genentech Inc. Confidential

Acknowledgements q Genentech 4 Ian Fleming 4 Lauren Haworth 4 Sandra Minjoe 4 Rajkumar Sharma 4 Peggy Wooster 4 Susan Zhao q I 3 Statprobe 4 Chakrapani Kolluru q Pharma. Stat 4 John Brega 4 Jane Diefenbach 38 Genentech Inc. Confidential

Contacts Patricia L. Gerend Chris Price Senior Manager, Statistical Programming & Analysis Senior Programmer Roche Products Limited Genentech, Inc. Welwyn Garden City, UK South San Francisco, California, USA chris. price. cp 1@roche. com gerend@gene. com + 44 (0)1707 36 5801 650 -225 -6005 Michelle Zhang Olivier Leconte Senior Statistical Programmer Analyst Programming Team Leader Genentech, Inc. Roche Products Limited South San Francisco, CA, USA Welwyn Garden City, UK zhang@gene. com olivier. leconte@roche. com 650 -225 -7414 +44 (0) 1707 36 5710 39 Genentech Inc. Confidential

Questions? 40 Genentech Inc. Confidential