CDISC Controlled Terminology Initiative An Overview 1 June















- Slides: 27

CDISC Controlled Terminology Initiative: An Overview 1 June 2007 Bron W. Kisler CDISC Co-Founder Terminology Program Director bkisler@cdisc. org

CDISC Terminology Initiative § Overview § Terminology for SDTM § NCI EVS Partnership § The Home Stretch…

CDISC Terminology • Formalized CDISC Terminology Initiative in 2005 • Primary Objective: to define and support the terminology needs of the CDISC models across the clinical trial continuum (SDTM ↔ CDASH) • Terminology Initiative is comprised of 50+ team members (Global Sponsors, Regulatory, Academic Institutions, etc. ) distributed across 4 project teams • Key partnership with the US National Cancer Institute Enterprise Vocabulary Services (NCI EVS) • Combined RCRIM Vocabulary & CDISC Terminology teams to ensure common development SDTM = Study Data Tabulation Model (e. Submissions) CDASH = Clinical Data Acquisition Standards Harmonization

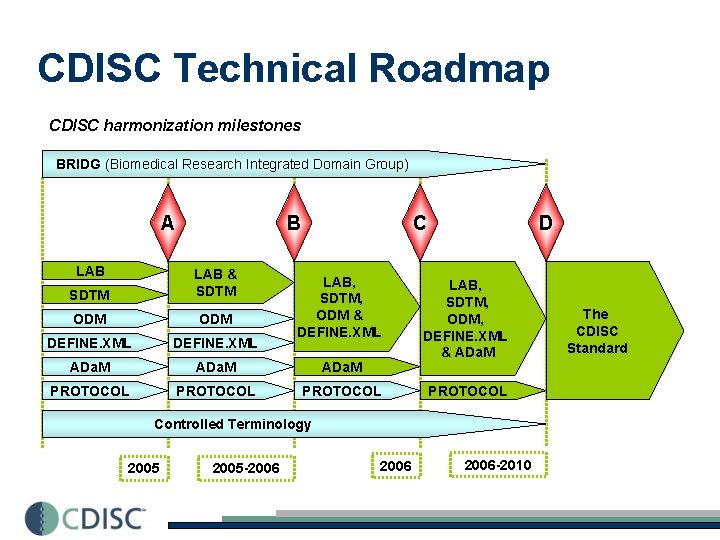
CDISC Technical Roadmap CDISC harmonization milestones BRIDG (Biomedical Research Integrated Domain Group) A LAB B C SDTM LAB & SDTM ODM DEFINE. XML ADa. M PROTOCOL LAB, SDTM, ODM & DEFINE. XML D LAB, SDTM, ODM, DEFINE. XML & ADa. M PROTOCOL Controlled Terminology 2005 -2006 -2010 The CDISC Standard

The mission of CDISC is to develop and support global, platform-independent data standards that enable information system interoperability to improve medical research and related areas of healthcare

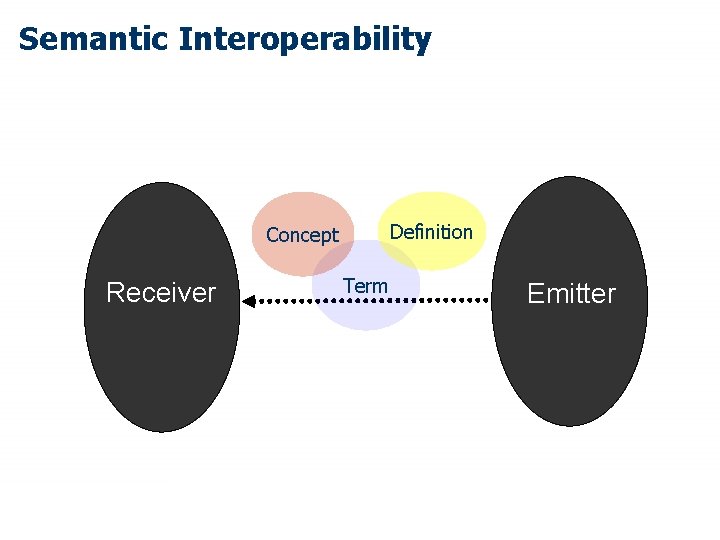
Interoperability: Ability of two or more systems or components to: 1. Exchange information (Syntactic Interoperability) and… 2. Predictably use the information that has been exchanged (Semantic Interoperability) Source: IEEE Standard Computer Dictionary

Semantic Interoperability ? Receiver SHELL… …shall we go now ? Emitter

Semantic Interoperability Definition Concept Receiver Term Emitter

Terminology for SDTM

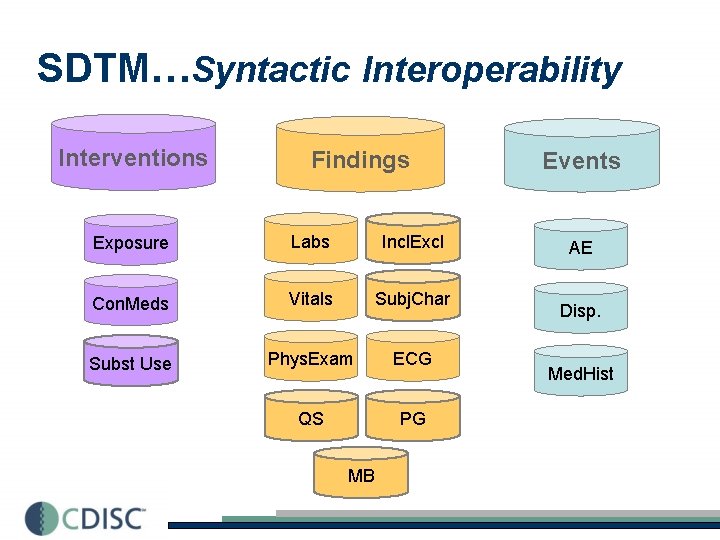
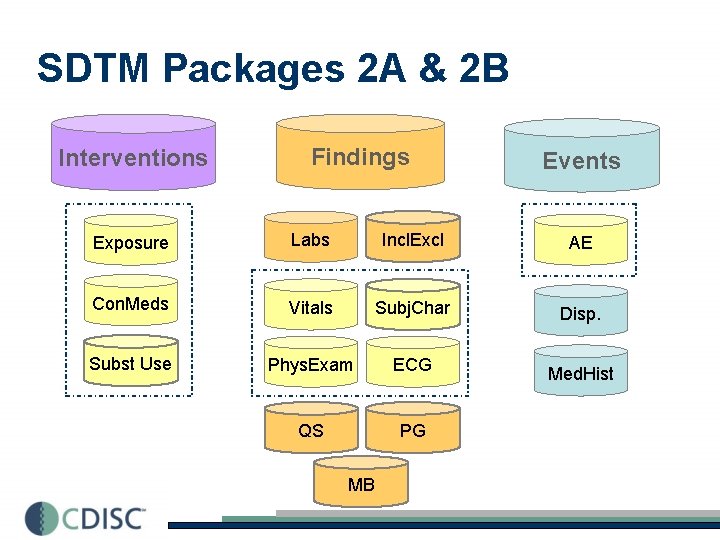
SDTM…Syntactic Interoperability Interventions Findings Exposure Labs Incl. Excl Con. Meds Vitals Subj. Char Subst Use Phys. Exam ECG QS PG MB Events AE Disp. Med. Hist

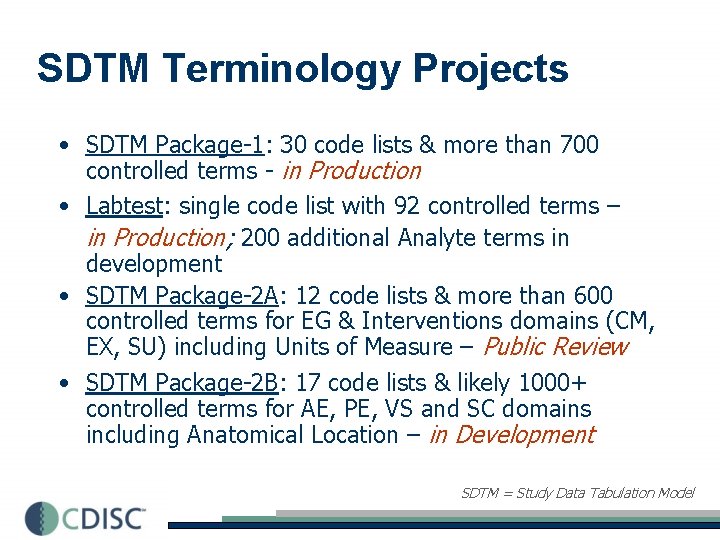
SDTM Terminology Projects • SDTM Package-1: 30 code lists & more than 700 controlled terms - in Production • Labtest: single code list with 92 controlled terms – in Production; 200 additional Analyte terms in development • SDTM Package-2 A: 12 code lists & more than 600 controlled terms for EG & Interventions domains (CM, EX, SU) including Units of Measure – Public Review • SDTM Package-2 B: 17 code lists & likely 1000+ controlled terms for AE, PE, VS and SC domains including Anatomical Location – in Development SDTM = Study Data Tabulation Model

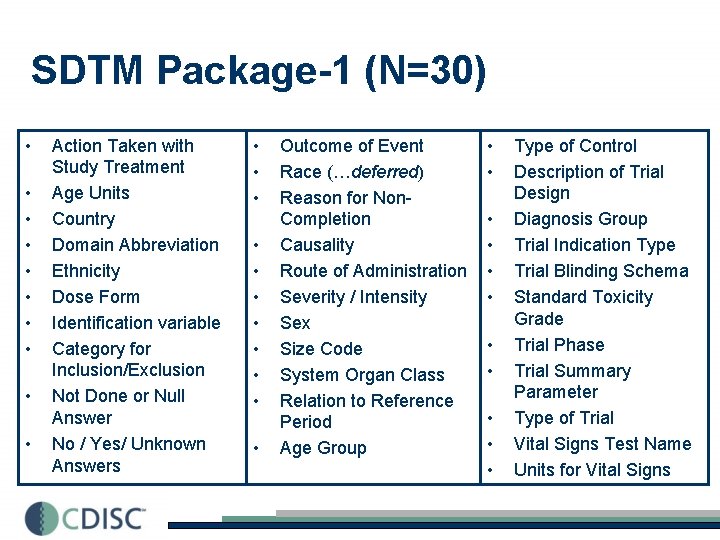
SDTM Package-1 (N=30) • • • Action Taken with Study Treatment Age Units Country Domain Abbreviation Ethnicity Dose Form Identification variable Category for Inclusion/Exclusion Not Done or Null Answer No / Yes/ Unknown Answers • • • Outcome of Event Race (…deferred) Reason for Non. Completion Causality Route of Administration Severity / Intensity Sex Size Code System Organ Class Relation to Reference Period Age Group • • • Type of Control Description of Trial Design Diagnosis Group Trial Indication Type Trial Blinding Schema Standard Toxicity Grade Trial Phase Trial Summary Parameter Type of Trial Vital Signs Test Name Units for Vital Signs

SDTM Packages 2 A & 2 B Interventions Findings Events Exposure Labs Incl. Excl AE Con. Meds Vitals Subj. Char Disp. Subst Use Phys. Exam ECG Med. Hist QS PG MB

Proposed Rule The Food and Drug Administration is proposing to amend the regulations governing the format in which clinical study data and bioequivalence data are required to be submitted for new drug applications (NDAs), biological license applications (BLAs), and abbreviated new drug applications (ANDAs). The proposal would revise our regulations to require that data submitted for NDAs, BLAs, and ANDAs, and their supplements and amendments be provided in an electronic format that FDA can process, review, and archive. The proposal would also require the use of standardized data structure, terminology, and code sets contained in current FDA guidance (the Study Data Tabulation Model (SDTM) developed by the Clinical Data Interchange Standards Consortium) to allow for more efficient and comprehensive data review. Federal Register / Volume 71, No. 237 / Monday, December 11, 2006

NCI EVS Partnership

Working Principals • Evaluate and/or utilize existing terminology 1 st • Expand existing vocabularies where incomplete, working with vocabulary developer / owner • Harmonize across CDISC Models and with preexisting vocabulary initiatives • Ensure terminology recommendations suit international needs for global organizations and projects • Ensure a sustainable “open source” environment for production terminology supporting terminology evolution

The Solution…NCI EVS NCI Enterprise Vocabulary Services (EVS) has committed expertise and significant resources in support of the CDISC Terminology Initiative and FDA Vocabulary Initiatives such as: SPL, ICSR, RPS, CDRH Healthcare Devices, etc… CDRH = US Center for Devices & Radiological Health SPL = Structured Product Label RPS = Regulated Product Submission

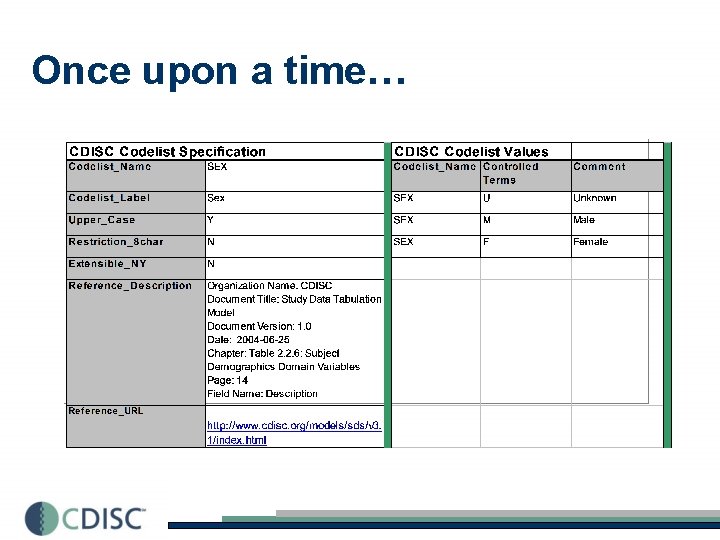
Once upon a time…

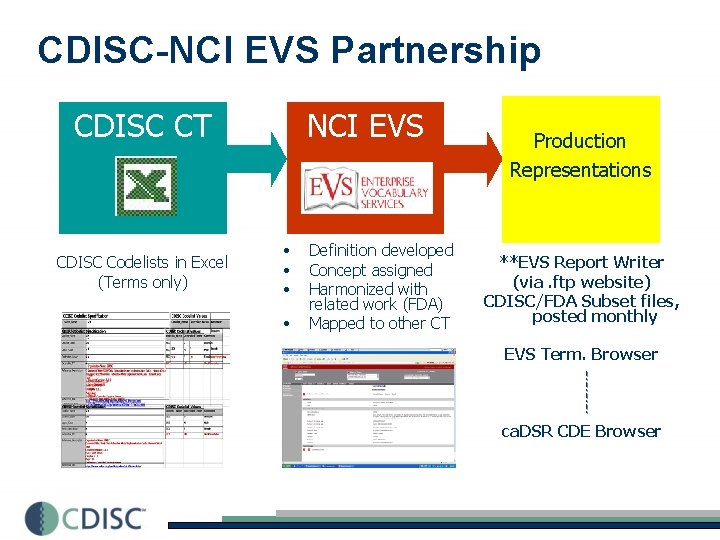
CDISC-NCI EVS Partnership CDISC CT CDISC Codelists in Excel (Terms only) NCI EVS • • Definition developed Concept assigned Harmonized with related work (FDA) Mapped to other CT Production Representations **EVS Report Writer (via. ftp website) CDISC/FDA Subset files, posted monthly EVS Term. Browser ca. DSR CDE Browser

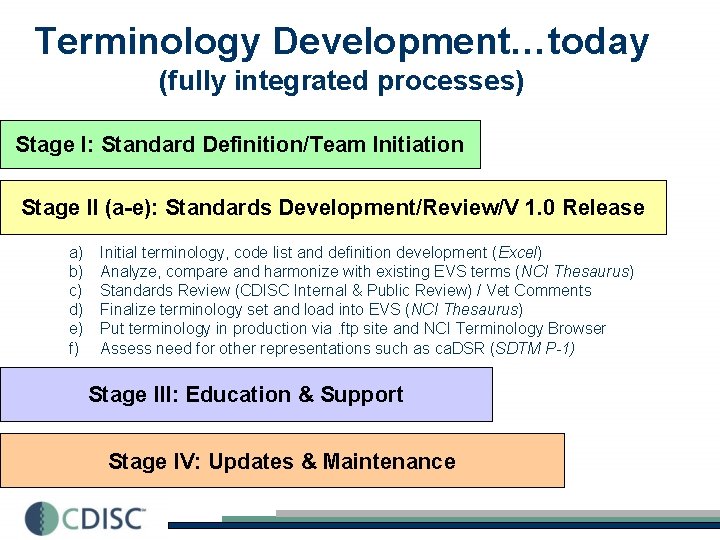
Terminology Development…today (fully integrated processes) Stage I: Standard Definition/Team Initiation Stage II (a-e): Standards Development/Review/V 1. 0 Release a) b) c) d) e) f) Initial terminology, code list and definition development (Excel) Analyze, compare and harmonize with existing EVS terms (NCI Thesaurus) Standards Review (CDISC Internal & Public Review) / Vet Comments Finalize terminology set and load into EVS (NCI Thesaurus) Put terminology in production via. ftp site and NCI Terminology Browser Assess need for other representations such as ca. DSR (SDTM P-1) Stage III: Education & Support Stage IV: Updates & Maintenance

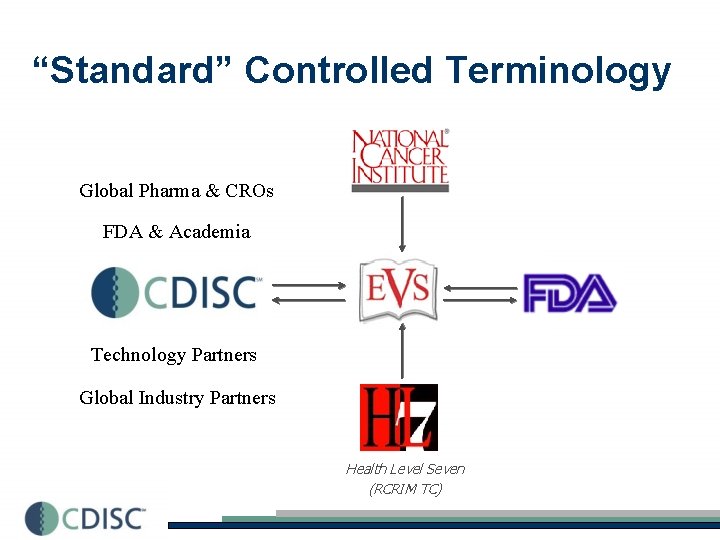
“Standard” Controlled Terminology Global Pharma & CROs FDA & Academia Technology Partners Global Industry Partners Health Level Seven (RCRIM TC)

The Home Stretch

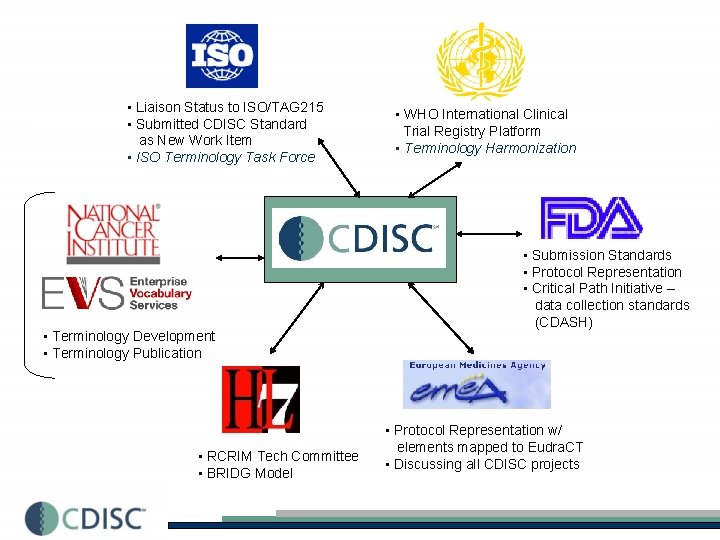
• Liaison Status to ISO/TAG 215 • Submitted CDISC Standard as New Work Item • ISO Terminology Task Force • Terminology Development • Terminology Publication • RCRIM Tech Committee • BRIDG Model • WHO International Clinical Trial Registry Platform • Terminology Harmonization • Submission Standards • Protocol Representation • Critical Path Initiative – data collection standards (CDASH) • Protocol Representation w/ elements mapped to Eudra. CT • Discussing all CDISC projects

2007 Terminology Priorities • Finalize production release of Labtest, SDTM Package 2 A and SDTM Package 2 B term sets • Harmonize with and support new global terminology projects such as those initiated by ISO, ICH & WHO • Develop user guidelines for production terminology access, download and implementation • Support priority projects such as developing global therapeutic data standards for Tuberculosis & Cardiology • Scope and support important future projects – CDASH, BRIDG, SEND and beyond • Further develop terminology education and training, including workshops and online tutorials CDASH = Clinical Data Acquisition Standards Harmonization BRIDG = Biomedical Research Integrated Domain Group

Strategy Moving Forward • Continue to increase team membership (65+ in 2007) with broader international coverage • Further refine terminology development / production processes for faster release cycles • Be deliberate in developing new leaders with focus on CDISC Terminology Organization • Expand improve cross team communication (Glossary Group, new SDS initiatives, CDASH) • Improve cross-fertilization with HL 7 RCRIM projects and encourage “dual citizenship” RCRIM = Regulated Clinical Research Information Management CDASH = Clinical Data Acquisition Standards Harmonization

Special Thanks to… Andreas Gromen (Schering AG, Germany) Margaret Haber (NCI EVS) Mary Lenzen (Octagon Research) CDISC Terminology Team Volunteers CDISC is now in “Control” of its terminology
