CDISC based e Submission Key Points of the

CDISC based e. Submission Key Points of the CDISC SDTM/ADa. M Pilot Monika Kawohl Senior Statistical Programmer Accovion Gmb. H, Marburg, Germany CDISC German UG Meeting 02 -Sep-2008
Outline Coverage & Aim Process CDISC Model Specifics • SDTM • ADa. M • Define. xml FDA Reviewers‘ Feedback Summary and Conclusions CDISC German UG Meeting 02 -Sep-2008 2
Coverage Legacy data conversion of 1 study to SDTM domains (SAS V 5 XPT format) Annotated CRF ADa. M datasets created from SDTM Analysis Results based on ADa. M datasets All tied together via Metadata in define. xml e. CTD structure but PDF TOC files CDISC German UG Meeting 02 -Sep-2008 3
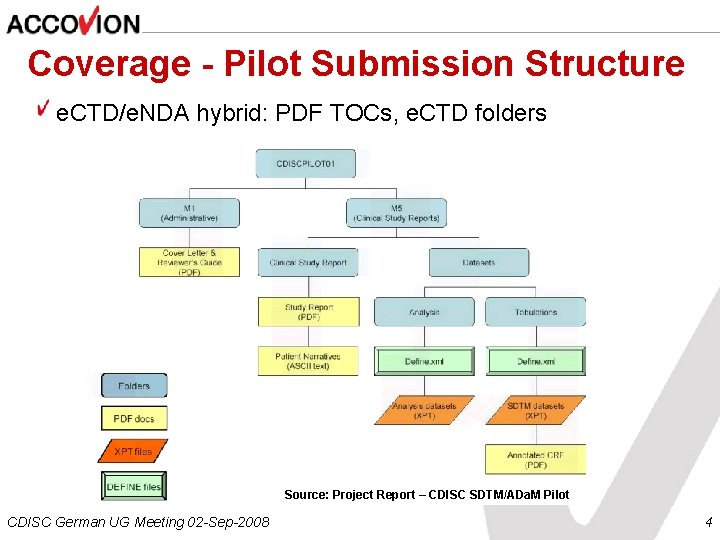
Coverage - Pilot Submission Structure e. CTD/e. NDA hybrid: PDF TOCs, e. CTD folders Source: Project Report – CDISC SDTM/ADa. M Pilot CDISC German UG Meeting 02 -Sep-2008 4
Aim Verify interoperability of CDISC standards Obtain FDA reviewers‘ feedback Provide implementation example Gap analysis of single CDISC Standards But. . . Not intended as guidance regarding • Contents • Process applied CDISC German UG Meeting 02 -Sep-2008 5

Process Source: Webcast – Results and Lessons from the CDISC SDTM/ADa. M Pilot Project CDISC German UG Meeting 02 -Sep-2008 6
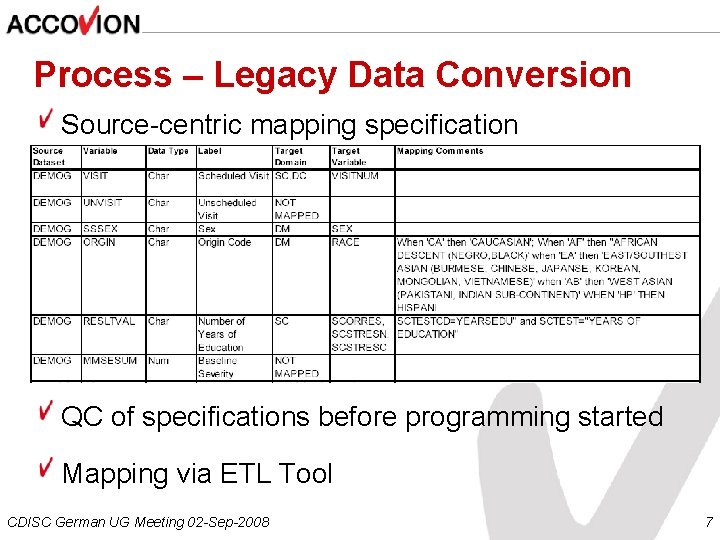
Process – Legacy Data Conversion Source-centric mapping specification QC of specifications before programming started Mapping via ETL Tool CDISC German UG Meeting 02 -Sep-2008 7
Process – SDTM/ADa. M/define. xml SDTM “without“ derived => ADa. M => SDTM some derived • Facilitates usage of derived data in SDTM review tools • Lineage issue • Explorative use - final decision pending Definition of metadata at specification level • Prescriptive use of metadata reduces consistency issues à (Suite of SAS macros used available to CDISC members) • Concise computational algorithm descriptions à No programs were submitted CDISC German UG Meeting 02 -Sep-2008 8
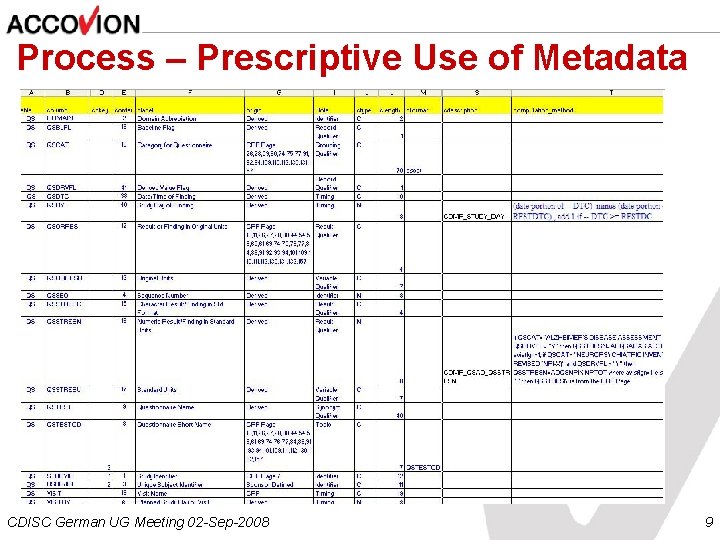
Process – Prescriptive Use of Metadata CDISC German UG Meeting 02 -Sep-2008 9
Process – Quality Control (QC) QC of specifications QC of programming/mapping against specifications • Option: independent re-programming QC of Analysis Results against SAP QC of define. xml • Verifying links and contents • Verifying of technical requirements via tools Consistency via automated process based on metadata CDISC German UG Meeting 02 -Sep-2008 10
CDISC Model Specifics CDISC SDTM IG V 3. 1. 1 CDISC SDTM V 1. 1 CDISC ADa. M • CDISC ADa. M IG was not yet available (in preparation) CDISC CRT-DDS (define. xml) V 1. 0 • CDISC ODM V 1. 3 for define. xml à define. xml V 1. 0 standard based on ODM V 1. 2 • Custom stylesheet • Schema extension for ADa. M part CDISC German UG Meeting 02 -Sep-2008 11
CDISC SDTM Specifics SDTM “without derived“ • Includes SDTM required derived variables, e. g. , USUBJID, --BLFL, (--DY) SDTM “with some derived“ • Purpose: viewing derived data in standard domain structures • Includes data derived in the ADa. M creation step à Population Flags (SUPPDM) à AE Treatment Emergent Flag and Med. DRA variables (SUPPAE) à Endpoint Flag and Result Divided by UL of Normal Range (SUPPLB) à Total Score as sum of single scores (New record in QS) • ADa. M => SDTM ensures consistency of derivation results CDISC German UG Meeting 02 -Sep-2008 12
CDISC ADa. M Specifics Important since SDTM datasets not analysis-ready ADSL concept of ADa. M V 2. 0 • ADSL very helpful for all reviewers à Baseline Characteristics à Treatment Variables à Population Flags à Patient à Start Completion Status and End of Study Drug, Date Patient was Last Observed All analysis datasets instead of datasets for key analyses à At least Analysis Metadata could be limited to key results CDISC German UG Meeting 02 -Sep-2008 13
CDISC ADa. M Specifics (Continued) Tracebility and transparency of analysis datasets • Clear lineage from CRF to analysis à Clear, unambigous metadata (computation methods) à Include all CRF data + derived data à Appropriate data selection via flags • Facilitate reproduction of analysis results • Allow for sensitivity analysis, test of robustness Sponsor-specific standards, e. g. variable names, PARAMs NOW available: Draft ADa. M IG CDISC German UG Meeting 02 -Sep-2008 14
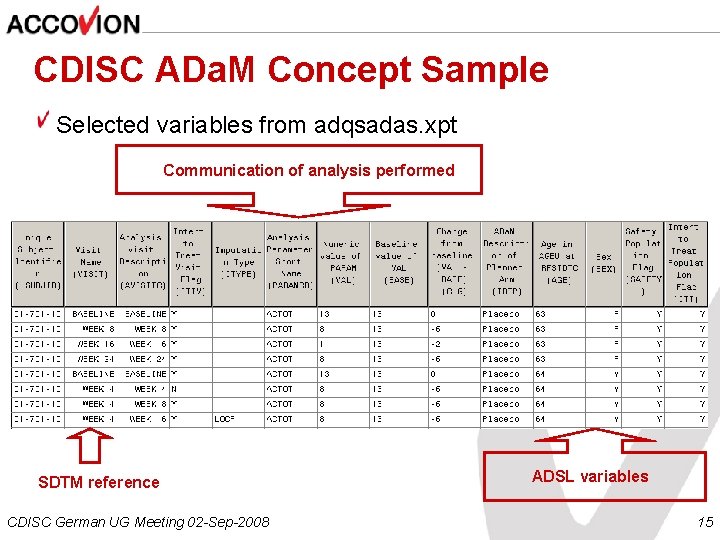
CDISC ADa. M Concept Sample Selected variables from adqsadas. xpt Communication of analysis performed SDTM reference CDISC German UG Meeting 02 -Sep-2008 ADSL variables 15
CDISC define. xml Specifics SDTM and ADa. M metadata included in 1 define. xml file • Facilitate links between SDTM and ADa. M and vice versa • Define. xml included in tabulations and analysis e. CTD folder Navigation improved (define. pdf comparable) • Framed version solves back button issue (IE specific) CDISC German UG Meeting 02 -Sep-2008 16
CDISC define. xml Specifics (Continued) Valuable sample for Analysis Metadata • List of Analysis Results • Standardized results description incl. links to à Analysis output, underlying dataset, specifications (e. g. SAP) Layout not 100% consistent with new Draft ADa. M documents CDISC German UG Meeting 02 -Sep-2008 17
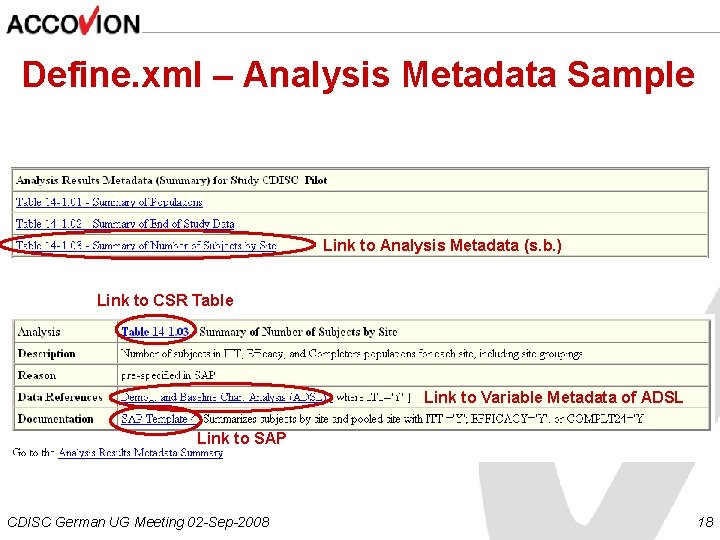
Define. xml – Analysis Metadata Sample Link to Analysis Metadata (s. b. ) Link to CSR Table Link to Variable Metadata of ADSL Link to SAP CDISC German UG Meeting 02 -Sep-2008 18
CDISC define. xml Specifics (continued) Deviations from Draft Metadata Submission Guidelines • All derivations described via computation methods à Separate column • Comment column reserved for ADa. M links to source datasets • No Variable Value Level Metadata à Limited to Controlled Terminology for test codes and names • Controlled Terminology representation subdivided in à Code Lists (Code and Decode) à Discrete Value Listings Printing Issue CDISC German UG Meeting 02 -Sep-2008 19
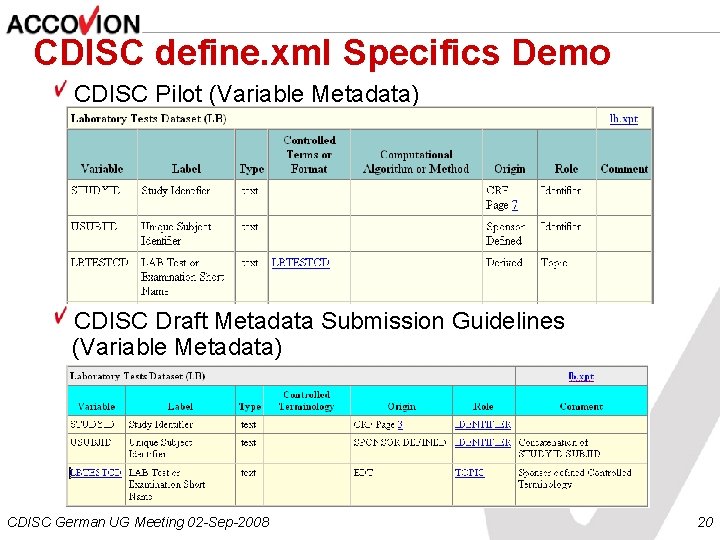
CDISC define. xml Specifics Demo CDISC Pilot (Variable Metadata) CDISC Draft Metadata Submission Guidelines (Variable Metadata) CDISC German UG Meeting 02 -Sep-2008 20
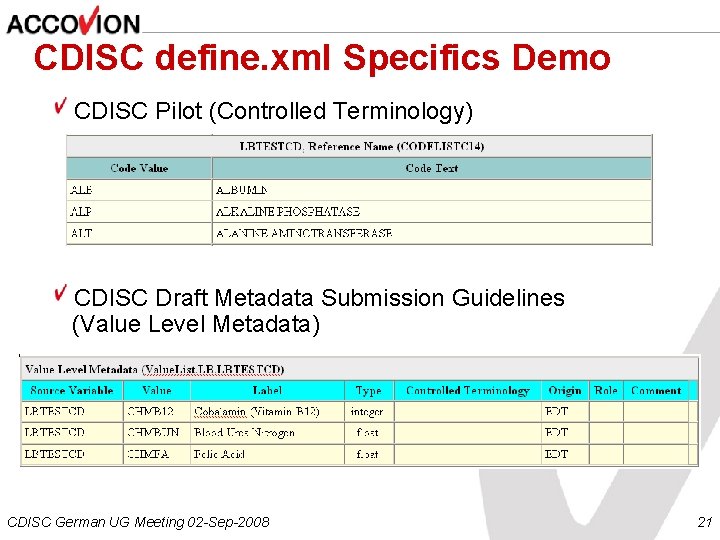
CDISC define. xml Specifics Demo CDISC Pilot (Controlled Terminology) CDISC Draft Metadata Submission Guidelines (Value Level Metadata) CDISC German UG Meeting 02 -Sep-2008 21
FDA Reviewers‘ Feedback Standard disclaimer (NOT the general FDA view) Overall, data were suitable for reviewers‘ needs Value of SDTM depends on reviewers‘ training status • Use define. xml comments to explain “CDISC jargon“ Integration of Reviewer‘s Guide recommended ADa. M essential since SDTM not Analysis-Ready • ADSL variables, e. g. treatment in each dataset Derived variables in SDTM helpful • Use of SDTM based review tools • CAVEAT: Results may not be reproducible based on sole SDTM (additional analysis flags to be considered) CDISC German UG Meeting 02 -Sep-2008 22
FDA Reviewers‘ Feedback (continued) Use of SDTM Review Tool Web. SDM • SDTM compliance check • SDTM/define. xml consistency check • Graphical Patient Profiles • Merge of core variables (e. g. sex, treatment) to domains • Merge of SUPPQUAL to parent domains • Export of combined data to other tools Reviewers at time of Pilot more familiar with other tools CDISC German UG Meeting 02 -Sep-2008 23

FDA Reviewers‘ Feedback (continued) Data documentation via define. xml • Concept, Content, Navigation, Reviewer‘s Guide • Printing Sample for multiple-studies submission is missing FDA committed to standards Success of SDTM/ADa. M depends on • Training (implementers and reviewers) • Experience (implementers and reviewers) • Availability of suitable review tools CDISC German UG Meeting 02 -Sep-2008 24
Summary and Conclusions Overall, data were suitable for reviewers‘ needs Very helpful implementation example No guidance => evolution of CDISC standards Importance of ADa. M when submitting SDTM Familiarity with CDISC models is a key factor • Implementer • Reviewer Watch out for next pilot: multiple-studies safety data CDISC German UG Meeting 02 -Sep-2008 25

References CDISC SDTM/ADa. M Pilot Project – Project Report (SDTMADa. MPilot. Project. Report. pdf) Slides from the CDISC Pilot Webcast (bmc_cdisc_sdtm-adam_pilot_v-2. pdf) Cathy Barrow‘s presentation at CDISC EU IC 2008 (CDISC presentations website) Revised Pilot Submission Package as provided by CDISC (CDISC Members Only website) CDISC German UG Meeting 02 -Sep-2008 26

Thank you! Questions? CDISC German UG Meeting 02 -Sep-2008
- Slides: 27