CDCs National Healthcare Safety Network NHSN and Its


















- Slides: 34

CDC’s National Healthcare Safety Network (NHSN) and Its Use in Mandatory Reporting of HAI Daniel A. Pollock Surveillance Branch Division of Healthcare Quality Promotion Texas Healthcare-Associated Infection (HAI) Prevention Conference October 26, 2010 National Center for Emerging and Zoonotic Infectious Diseases Division of Healthcare Quality Promotion


U. S. Standard Death Certificate Cause of Death Section

Medical Error: A Leading Cause of Death • An estimated 44, 000 – 98, 000 Americans die in hospitals each year as a result of medical errors • Even when using the lower estimate, deaths due to medical error rank as the seventh leading cause of deaths • The consequences of high error rates include hospital-acquired infections and other treatmentrelated infections Institute of Medicine report, November 1999

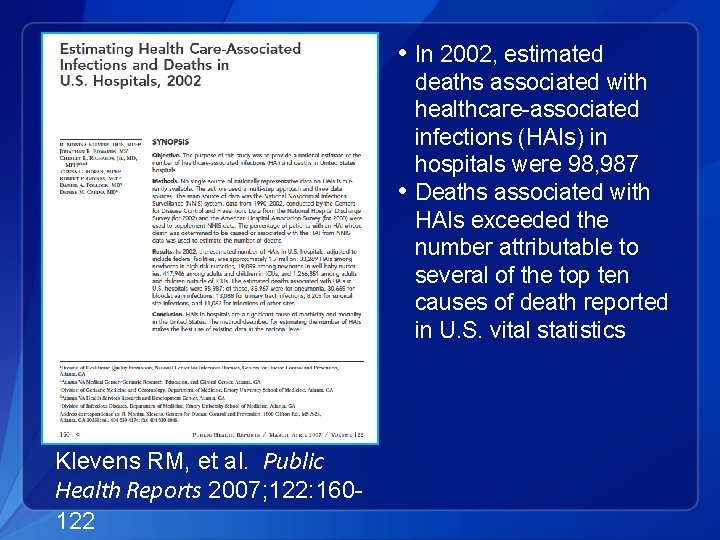
• In 2002, estimated deaths associated with healthcare-associated infections (HAIs) in hospitals were 98, 987 • Deaths associated with HAIs exceeded the number attributable to several of the top ten causes of death reported in U. S. vital statistics Klevens RM, et al. Public Health Reports 2007; 122: 160122

Objectives • NHSN overview– Purposes, operational features, and HAI reports • Use of NHSN for mandatory reporting – State mandates and the Center for Medicare and Medicaid Services (CMS) Inpatient Prospective Payment System (IPPS) rule • Challenges and opportunities – Near-term programmatic and operational priorities for CDC and partners

What a Difference 5+ Years Makes: HAI Reporting Evolves from Purely Voluntary and Confidential to Mainly Mandatory and Public • NHSN was launched in 2005 as a • • • voluntary and confidential system Approximately 300 hospitals participated initially In 2006, Vermont became the first state to mandate use of NHSN Rapid increase in users; over 3000 hospitals participate in 2010 22 states and the District of Columbia mandate use of NHSN Center for Medicare and Medicaid Services (CMS) will require hospital reporting via NHSN starting in 2011

NHSN in Brief • Designed for surveillance and prevention of healthcareassociated infections and other adverse events among patients and healthcare personnel and for process-of-care measurement and improvement • Technical infrastructure enables manual data entry into web-based application or electronic reporting through file imports or transfers • Serves multiple purposes and user groups: infection control, quality improvement, prevention research, mandatory public reporting, and public health surveillance

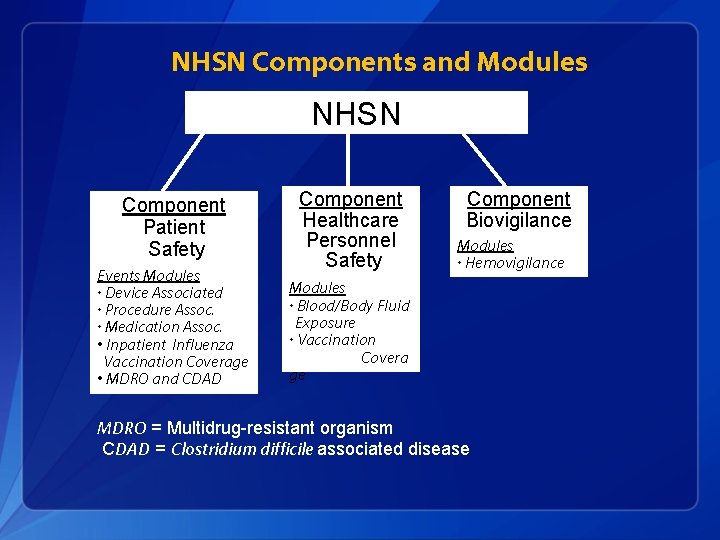
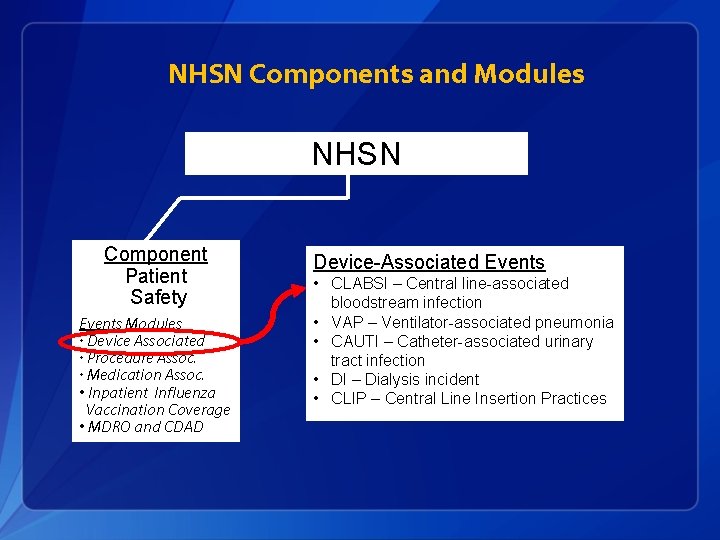
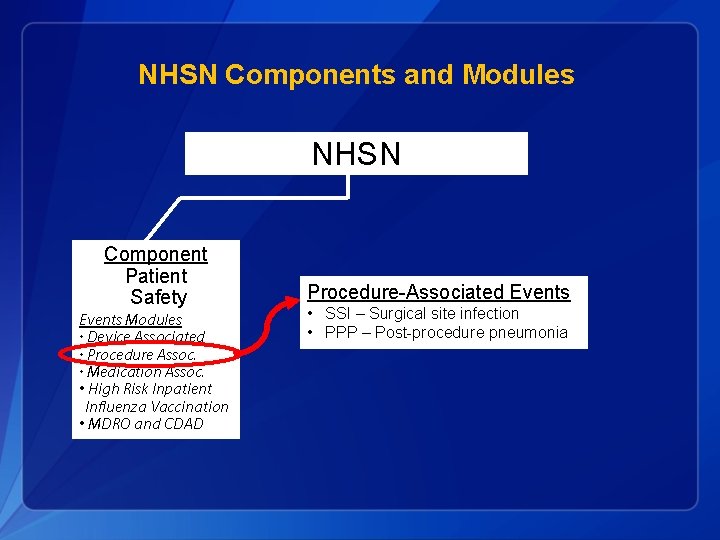
NHSN Components and Modules NHSN Component Patient Safety Events Modules • Device Associated • Procedure Assoc. • Medication Assoc. • Inpatient Influenza Vaccination Coverage • MDRO and CDAD Component Healthcare Personnel Safety Component Biovigilance Modules • Hemovigilance Modules • Blood/Body Fluid Exposure • Vaccination Covera ge MDRO = Multidrug-resistant organism CDAD = Clostridium difficile associated disease

NHSN Components and Modules NHSN Component Patient Safety Events Modules • Device Associated • Procedure Assoc. • Medication Assoc. • Inpatient Influenza Vaccination Coverage • MDRO and CDAD Device-Associated Events • CLABSI – Central line-associated bloodstream infection • VAP – Ventilator-associated pneumonia • CAUTI – Catheter-associated urinary tract infection • DI – Dialysis incident • CLIP – Central Line Insertion Practices

NHSN Components and Modules NHSN Component Patient Safety Events Modules • Device Associated • Procedure Assoc. • Medication Assoc. • High Risk Inpatient Influenza Vaccination • MDRO and CDAD Procedure-Associated Events • SSI – Surgical site infection • PPP – Post-procedure pneumonia

NHSN Protocol and Data Collection Form

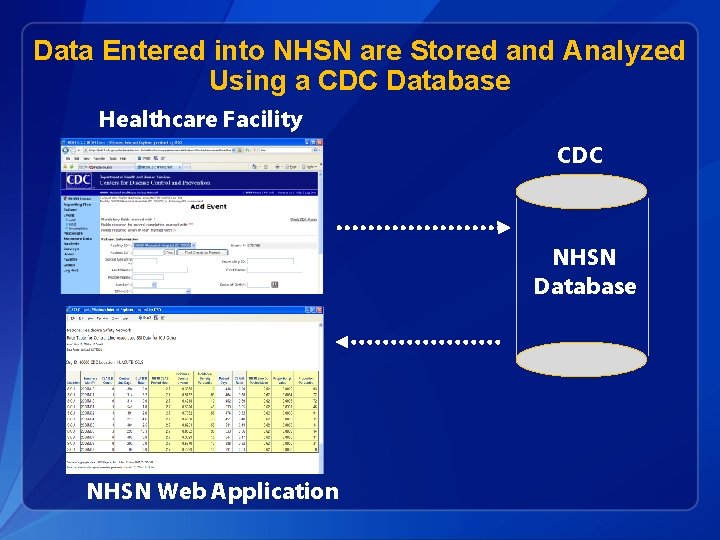
Data Entered into NHSN are Stored and Analyzed Using a CDC Database Healthcare Facility CDC NHSN Database NHSN Web Application

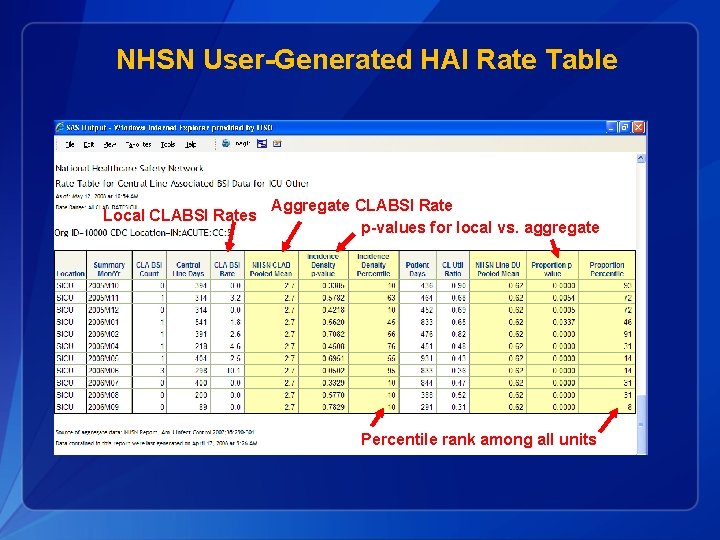
NHSN User-Generated HAI Rate Table Local CLABSI Rates Aggregate CLABSI Rate p-values for local vs. aggregate Percentile rank among all units

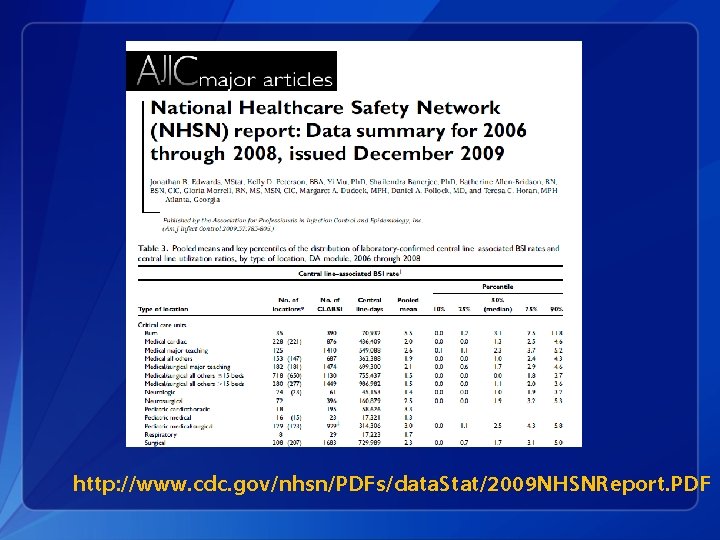
http: //www. cdc. gov/nhsn/PDFs/data. Stat/2009 NHSNReport. PDF

http: //www. cdc. gov/hai/pdfs/stateplans/SIR_05_25_2010. pdf

NHSN and Groups: Enabling States and Other Group Users to Gain Data Access • A Group is a collection of healthcare facilities that have joined together within the NHSN framework to enable a third party other than themselves or CDC to gain access to some or all of their data at a single (Group) level for a specific purpose, e. g. , state reporting mandate, prevention collaborative, research project • Any third party entity can form a Group, e. g. , state health department, quality improvement organization, hospital system • Facilities may join multiple Groups

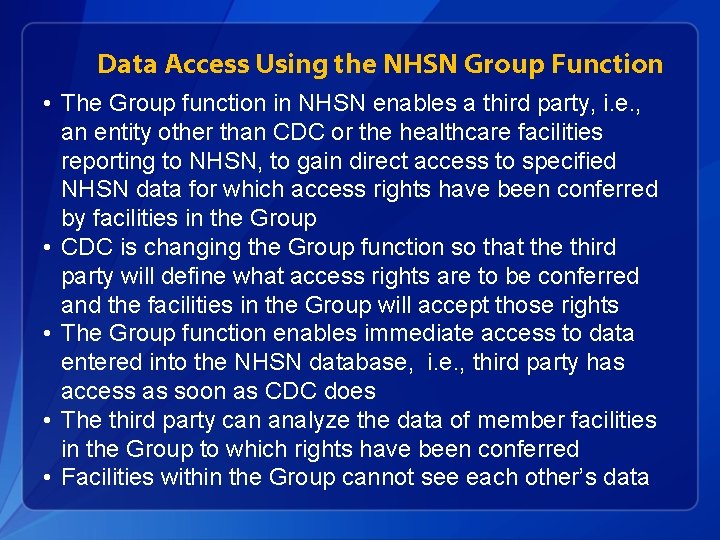
Data Access Using the NHSN Group Function • The Group function in NHSN enables a third party, i. e. , an entity other than CDC or the healthcare facilities reporting to NHSN, to gain direct access to specified NHSN data for which access rights have been conferred by facilities in the Group • CDC is changing the Group function so that the third party will define what access rights are to be conferred and the facilities in the Group will accept those rights • The Group function enables immediate access to data entered into the NHSN database, i. e. , third party has access as soon as CDC does • The third party can analyze the data of member facilities in the Group to which rights have been conferred • Facilities within the Group cannot see each other’s data

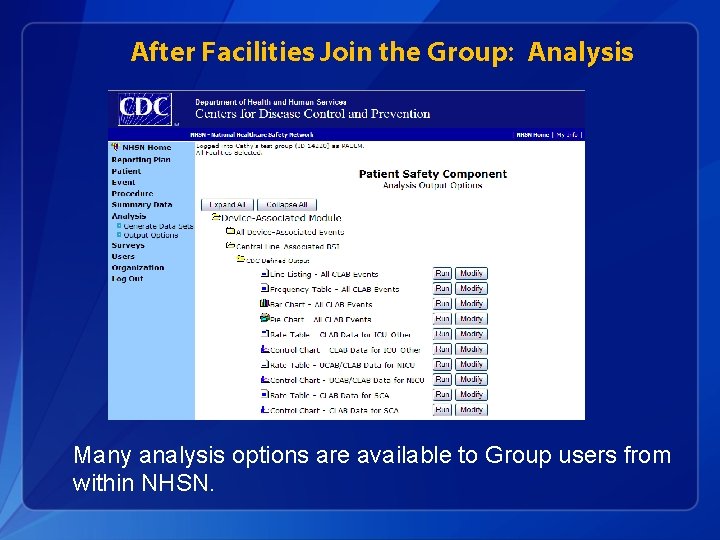
After Facilities Join the Group: Analysis Many analysis options are available to Group users from within NHSN.

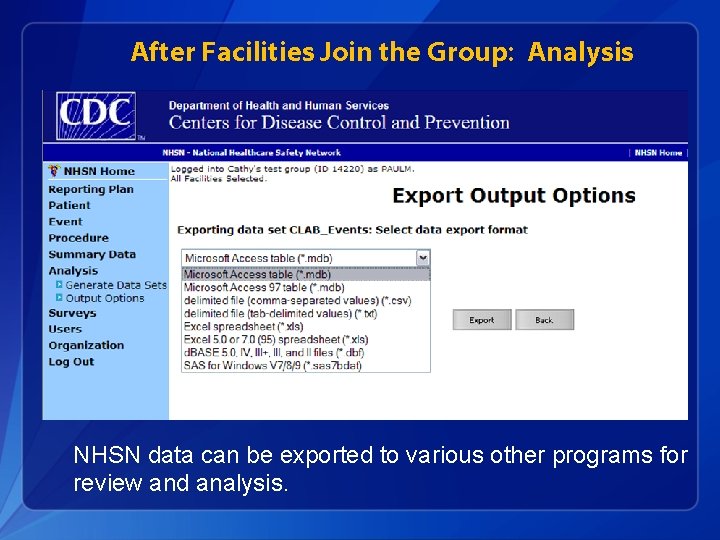
After Facilities Join the Group: Analysis NHSN data can be exported to various other programs for review and analysis.

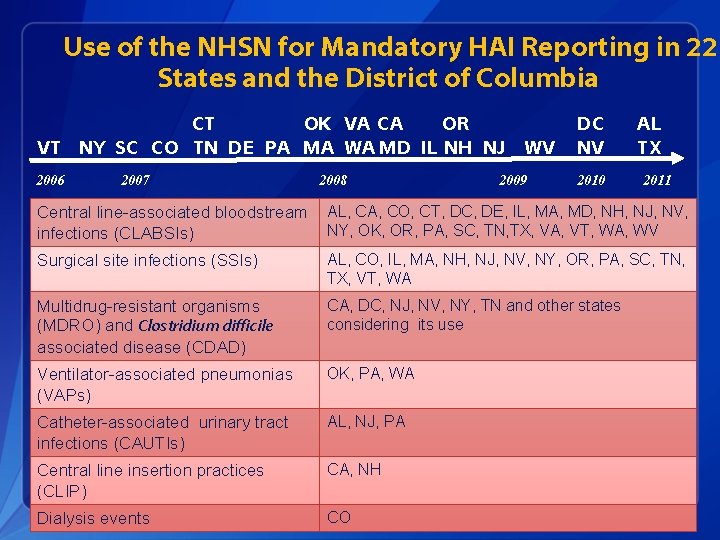
Use of the NHSN for Mandatory HAI Reporting in 22 States and the District of Columbia CT OK VA CA OR VT NY SC CO TN DE PA MA WA MD IL NH NJ WV DC NV 2006 2010 2007 2008 2009 AL TX 2011 Central line-associated bloodstream infections (CLABSIs) AL, CA, CO, CT, DC, DE, IL, MA, MD, NH, NJ, NV, NY, OK, OR, PA, SC, TN, TX, VA, VT, WA, WV Surgical site infections (SSIs) AL, CO, IL, MA, NH, NJ, NV, NY, OR, PA, SC, TN, TX, VT, WA Multidrug-resistant organisms (MDRO) and Clostridium difficile associated disease (CDAD) CA, DC, NJ, NV, NY, TN and other states considering its use Ventilator-associated pneumonias (VAPs) OK, PA, WA Catheter-associated urinary tract infections (CAUTIs) AL, NJ, PA Central line insertion practices (CLIP) CA, NH Dialysis events CO

NHSN and the Inpatient Prospective Payment System (IPPS) Rule: Mandatory HAI Reporting on the Federal Level Begins in 2011 • Proposed rule - April 20, 2010 • Final rule published - August 16, 2010: http: //edocket. access. gpo. gov/2010/pdf/2010 -19092. pdf • First reporting quarter - January 1 through March 31, 2011 • First quarterly data due - August 15, 2011 Implementation of healthcare associated infection (HAI) reporting via NHSN as part of the IPPS rule is a work in progress – Central line association bloodstream infection (CLABSI) reporting is to begin in 2011. Surgical site infection (SSI) reporting is begin in 2012.

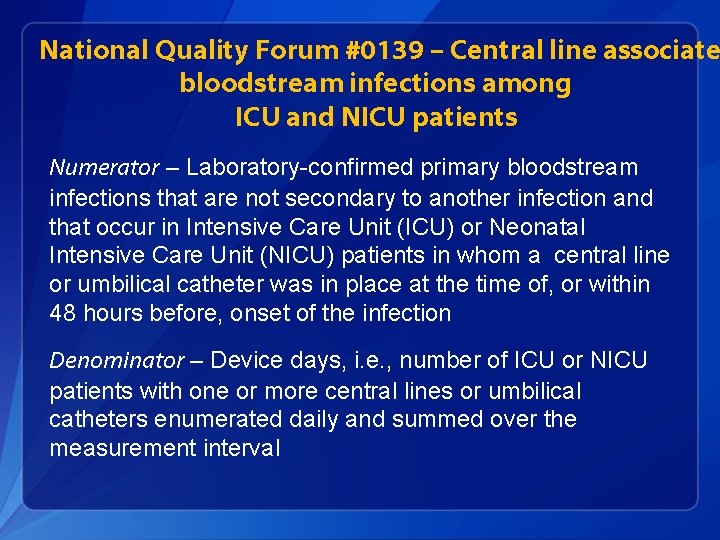
National Quality Forum #0139 – Central line associate bloodstream infections among ICU and NICU patients Numerator – Laboratory-confirmed primary bloodstream infections that are not secondary to another infection and that occur in Intensive Care Unit (ICU) or Neonatal Intensive Care Unit (NICU) patients in whom a central line or umbilical catheter was in place at the time of, or within 48 hours before, onset of the infection Denominator – Device days, i. e. , number of ICU or NICU patients with one or more central lines or umbilical catheters enumerated daily and summed over the measurement interval

National Quality Forum #0299 – Surgical site infections Numerator – Deep incisional or organ/space infections occurring within 30 days after an operative procedure* if no implant is in place or within 1 year if an implant is in place Denominator – Number of operative procedures* *Procedures in scope for the measure are coronary artery bypass graft and other cardiac surgery, hip or knee arthroplasty, colon surgery, hysterectomy (abdominal or vaginal), and vascular surgery

NHSN and the IPPS Rule: Operational Plans • Hospitals agree to participate by signing Annual Payment Update (APU) pledge form and NHSN consent agreement • CDC and CMS develop and use a common communications plan • New NHSN users take training prior to using the system • Hospitals new to NHSN complete enrollment process • Hospital CMS Certification Number (CCN) entered into NHSN, if not already entered • CMS provides CDC with a list of CCNs for hospitals participating in APU Hospital Inpatient Quality Reporting Program • CDC adds an NHSN analysis feature that enables quarterly calculation of hospital-specific CLABSI statistics • CDC submits CLABSI statistics to CMS using a secure Quality. Net exchange account • CMS uses hospital-specific statistics to pay hospitals that successfully report and for public reporting at the Hospital

NHSN Web Page Provides Training and Enrollment Resources for IPPS Participating Hospitals http: //www. cdc. gov/nhsn/cms-ipps-rule_training. html

New NHSN Consent Agreement • Healthcare facilities complete a NHSN Agreement to Participate and Consent form by downloading the document from a link sent to them, reading it, obtaining the required signatures, and returning the signed agreement to CDC • A new consent agreement will be available through the NHSN web-based application in late October or early November 2010 • Provisions added to the new agreement enable CDC to extend data access to state health departments, even in the absence of a mandate, and to CMS for quality measurement reporting requirements

NHSN and the IPPS Rule: Additional Operational Considerations • HAI reporting requirements for APU will co-exist with state HAI reporting requirements • Hospitals that use a commercial infection control software system can report HAI data electronically to NHSN • CLABSI data submitted via NHSN to comply with APU reporting requirements in 2011 will not be included in the CMS Hospital Inpatient Quality Reporting Program data validation

NHSN : Challenges and Opportunities • Leveraging electronic health record systems • Streamlining reporting • Setting national standards • Validating data • Informing practitioners, the public, and policymakers

What A Difference 10+ Years Makes: Learning from Medical Errors to Pay for Performance and Public Reporting Institute of Medicine report, November 1999 Health care reform, March 2010

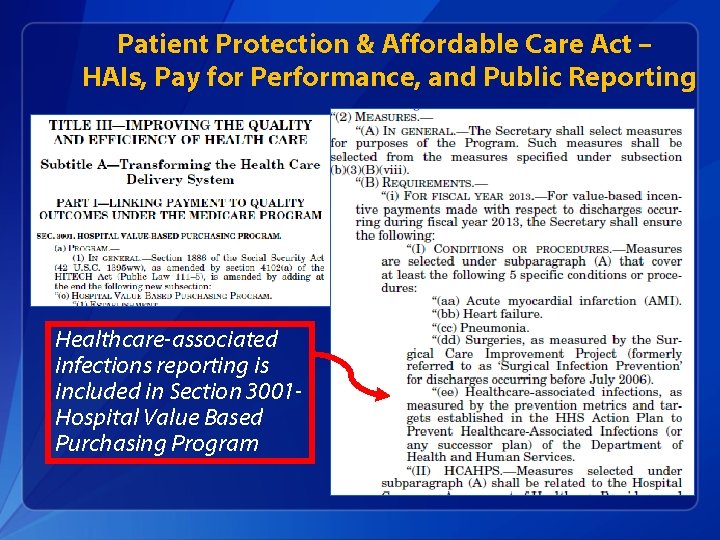
Patient Protection & Affordable Care Act – HAIs, Pay for Performance, and Public Reporting Healthcare-associated infections reporting is included in Section 3001 Hospital Value Based Purchasing Program

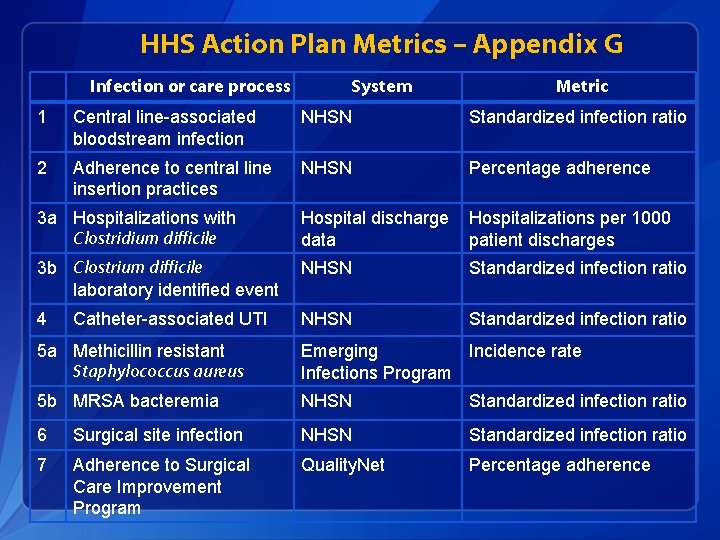
HHS Action Plan Metrics – Appendix G Infection or care process System Metric 1 Central line-associated bloodstream infection NHSN Standardized infection ratio 2 Adherence to central line insertion practices NHSN Percentage adherence 3 a Hospitalizations with Clostridium difficile Hospital discharge data Hospitalizations per 1000 patient discharges 3 b Clostrium difficile laboratory identified event NHSN Standardized infection ratio 4 NHSN Standardized infection ratio Catheter-associated UTI 5 a Methicillin resistant Staphylococcus aureus Emerging Incidence rate Infections Program 5 b MRSA bacteremia NHSN Standardized infection ratio 6 Surgical site infection NHSN Standardized infection ratio 7 Adherence to Surgical Care Improvement Program Quality. Net Percentage adherence


Thank You! Your Questions and Comments are Welcome For More Information about NHSN: http: //www. cdc. gov/nhsn/ National Center for Emerging and Zoonotic Infectious Diseases Division of Healthcare Quality Promotion