CDASH SDTM Mapping Kurt Hellstern German CDISC User


- Slides: 11

CDASH – SDTM Mapping Kurt Hellstern German CDISC User Group Meeting Berlin, 19. Feb. 2009

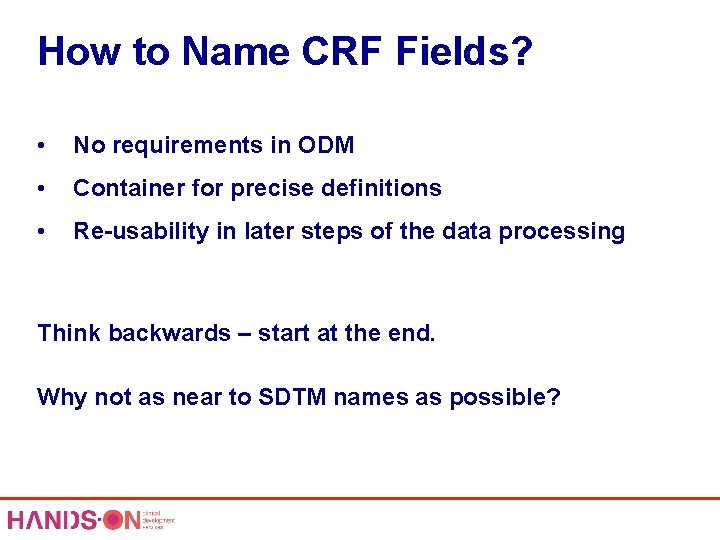
How to Name CRF Fields? • No requirements in ODM • Container for precise definitions • Re-usability in later steps of the data processing Think backwards – start at the end. Why not as near to SDTM names as possible?

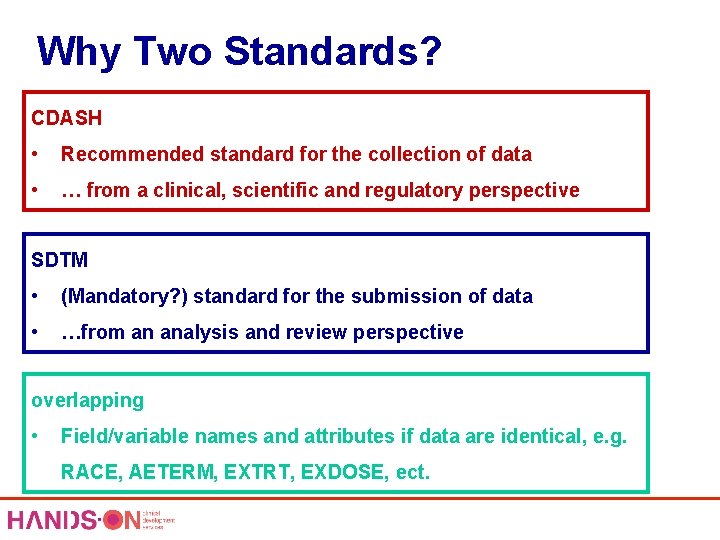
Why Two Standards? CDASH • Recommended standard for the collection of data • … from a clinical, scientific and regulatory perspective SDTM • (Mandatory? ) standard for the submission of data • …from an analysis and review perspective overlapping • Field/variable names and attributes if data are identical, e. g. RACE, AETERM, EXTRT, EXDOSE, ect.

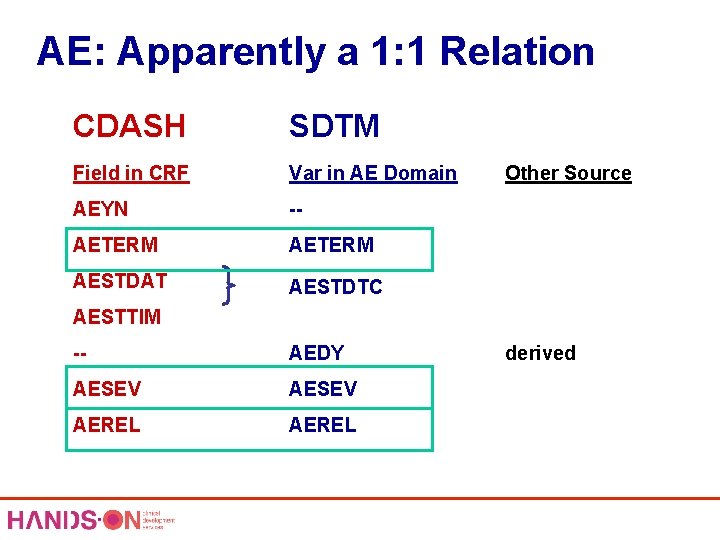
AE: Apparently a 1: 1 Relation CDASH SDTM Field in CRF Var in AE Domain AEYN -- AETERM AESTDAT AESTDTC CDASH Fields AESTTIM -- AEDY AESEV AEREL 1: 1 Other Source SDTM Variables derived

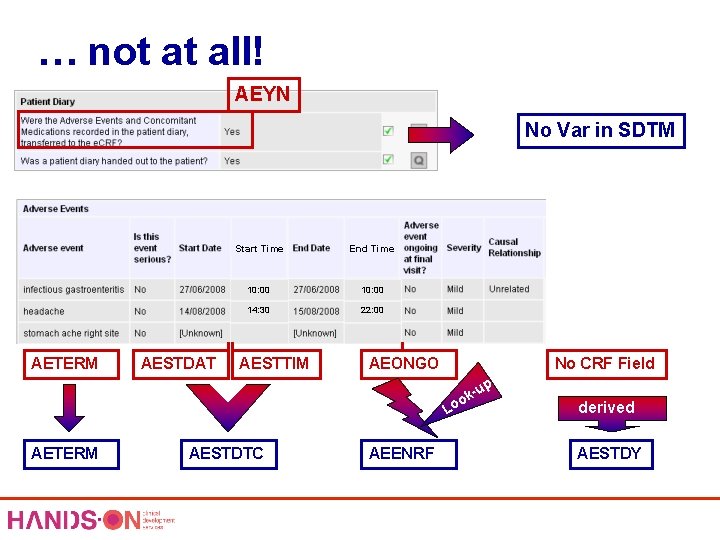
… not at all! AEYN No Var in SDTM AETERM AESTDAT Start Time End Time 10: 00 14: 30 22: 00 AESTTIM AEONGO No CRF Field -up k oo L AETERM AESTDTC AEENRF derived AESTDY

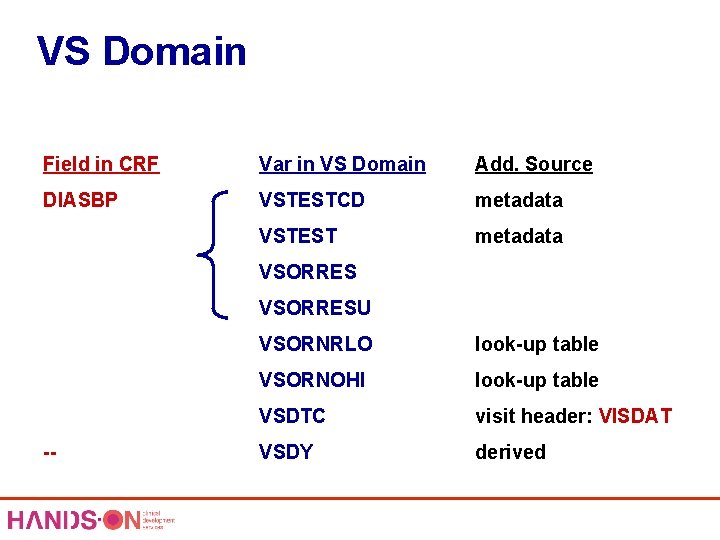
VS Domain Field in CRF Var in VS Domain Add. Source DIASBP VSTESTCD metadata VSTEST metadata VSORRESU -- VSORNRLO look-up table VSORNOHI look-up table VSDTC visit header: VISDAT VSDY derived

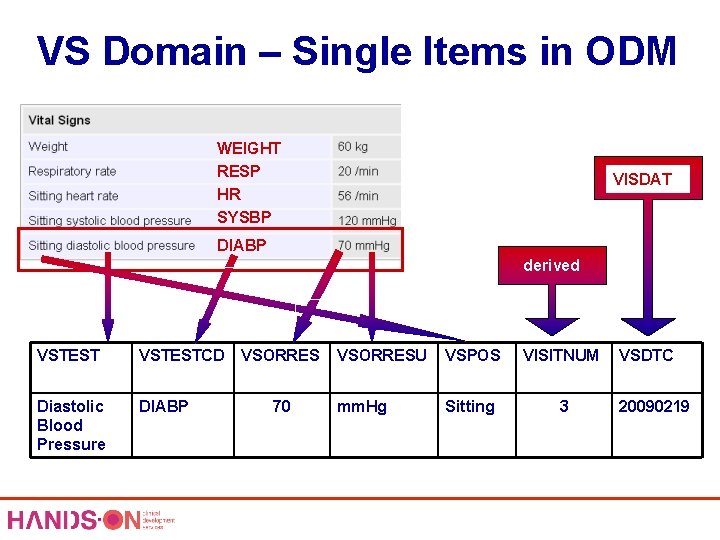
VS Domain – Single Items in ODM WEIGHT RESP HR SYSBP VISDAT DIABP derived VSTESTCD Diastolic Blood Pressure DIABP VSORRES 70 VSORRESU VSPOS VISITNUM mm. Hg Sitting 3 VSDTC 20090219

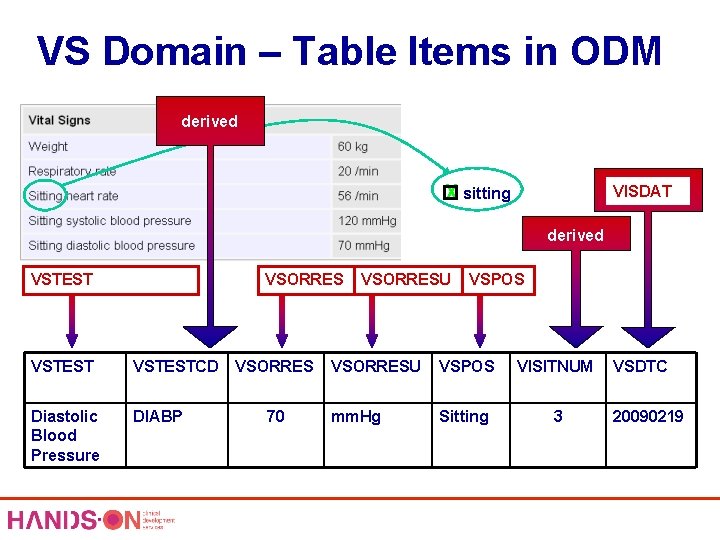
VS Domain – Table Items in ODM derived X sitting VISDAT derived VSTEST VSORRES VSTESTCD Diastolic Blood Pressure DIABP VSORRES 70 VSORRESU VSPOS VISITNUM mm. Hg Sitting 3 VSDTC 20090219

Other Domains? • Trial design: not much info entered on CRF’s • LB: How to load from labs using the LAB model • DA/EX: single tablet count in CRF • Detail in DM: USUBJID • etc.

Conclusion • Starting point for a metadata repository • Increasing clarity of our communication • Don’t hesitate to use CDASH, only beacause it does not match 100% your needs • Only practical experience brings us forwards

Thank you for your attention kurt. hellstern@hands-on. ch