CD 4 GLP Protocols Clinical Standard Operating Procedures













- Slides: 17

CD 4 GLP Protocols Clinical Standard Operating Procedures for CD 4 Enumeration by Flow Cytometry Using the BD FACSCount System Burt Houtz, CLS Technical Adviser, Flow Cytometry Applications BD Biosciences 080104

Laboratory Safety • Biohazard Safety • General Policies – – Approvals Waste disposal Accident reporting and follow-up Safety committee 2

Specimen Processing • General Policies – Approval – Periodic review • Specimen Collection – Phlebotomy • Training • Documentation • Specimen Handling and Transport • Specimen Requisition, Receipt and Assessment • Specimen Processing and Storage 3

Specimen Identification Log • Proper Identification • Condition of the Specimen • Date and Time – Collection – Receipt • Source – Lab location 4

Immunofluorescent Labeling • • • Preparing Controls Preparing Samples Vortexing Pipetting Incubation Times 5

FACSCount Quality Control • • Startup Procedure Priming the System Entering Control and Reagent Information Running Controls 6

FACSCount Quality Control Report • Control Run Result – Pass/Fail • CD 3 Range • CD 4 Range • Process Control Reporting 7

FACSCount Analysis of Specimens • • • Startup Procedure Priming the System Entering Patient and Reagent Information Running Patient Samples Cleaning Procedure Shutdown Procedure 8

Laboratory Reporting of CD 4 Test Results • Laboratory Worksheet • Recording Patient Test Results 9

FACSCount Maintenance Daily • Cleaning Monthly • Drain / Fill Cycle Annually • System Fluid Filter Change 10

FACSCount Maintenance Record • Recording Data • About Service Visits 11

CD 4 Enumeration Quality Assurance • Quality Control Procedures • Internal Controls – Evaluate Patient Data – T-Sum – CD 3 Consistency Check • Positive Reagent Control • Specimen Integrity • Reagent Quality Control 12

CD 4 Enumeration Quality Assurance • • • Reference Ranges Proficiency Testing Clerical Processing Quality Improvement Use of the Reagent Log 13

Data Reporting of CD 4 Test Results • • • Supervisory Review Interpret in the Proper Context Provide Appropriate Information Provide Legible Results Retain Files for a Sufficient Length of Time Notify the Physician If Results Are Below a Critical Level • Specify a Turnaround Time • Control Patient Data 14

Proficiency Testing • Formal Programs – CAP – NEQAS • Peer Review Processes • Country-Based Organizations • Specimen Concerns 15

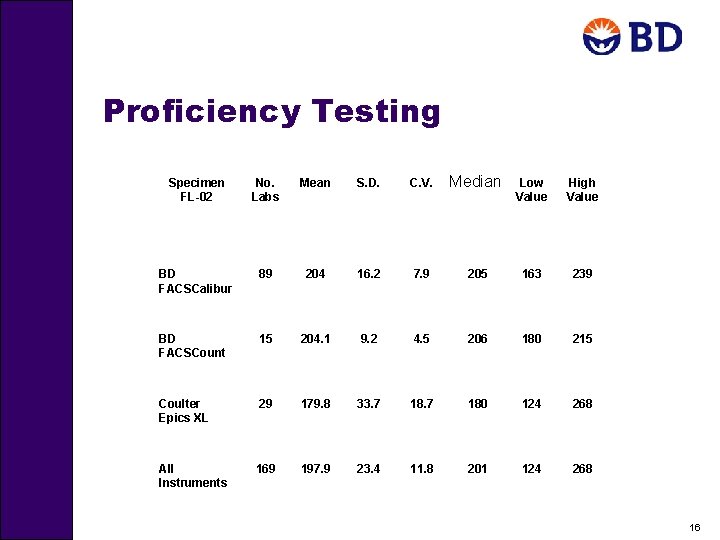
Proficiency Testing Specimen FL-02 No. Labs Mean S. D. C. V. Median Low Value High Value BD FACSCalibur 89 204 16. 2 7. 9 205 163 239 BD FACSCount 15 204. 1 9. 2 4. 5 206 180 215 Coulter Epics XL 29 179. 8 33. 7 180 124 268 All Instruments 169 197. 9 23. 4 11. 8 201 124 268 16

Clinical Training • • • What is it? Why it is Important Know the Audience (if you can!) Lesson Plan Feedback 17