Cavity ring down spectroscopy on C 6 H
![Previous Studies Gas Phase Absorption (440 -530 nm) [Porter & Ward] 2 B - Previous Studies Gas Phase Absorption (440 -530 nm) [Porter & Ward] 2 B -](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-3.jpg)
![Cavity Ring-Down Spectroscopy Loss = (2 al)(tc/2 L) Total loss = [(1 -R)+al] (tc/L) Cavity Ring-Down Spectroscopy Loss = (2 al)(tc/2 L) Total loss = [(1 -R)+al] (tc/L)](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-5.jpg)
![Long range scan [Huang] 7 Long range scan [Huang] 7](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-7.jpg)
![A”: 0. 209472(10) B”: 0. 186793(7) MW spec [Mc. Mahon] C”: 0. 098714988(20) B A”: 0. 209472(10) B”: 0. 186793(7) MW spec [Mc. Mahon] C”: 0. 098714988(20) B](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-9.jpg)
![12 B 1 X 2 A 1 - Energy Transfer Oscillator Strength [Kim] = 12 B 1 X 2 A 1 - Energy Transfer Oscillator Strength [Kim] =](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-13.jpg)


- Slides: 23

Cavity ring down spectroscopy on C 6 H 5 radical in a pulsed supersonic jet expansion discharge Keith Freel Dr. Michael Heaven Dr. M. C. Lin Dr. Joonbum Park 1

Phenyl C 6 H 5 Combustion Computational Benchmark (PAH Formation) Small Absorption Coefficient (in vis region) Astrophysics Environmental Impact 2
![Previous Studies Gas Phase Absorption 440 530 nm Porter Ward 2 B Previous Studies Gas Phase Absorption (440 -530 nm) [Porter & Ward] 2 B -](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-3.jpg)
Previous Studies Gas Phase Absorption (440 -530 nm) [Porter & Ward] 2 B - 2 A n p 1 1 Electron Spin Resonance [Bennett, Kasai] C 2 V symmetry Unpaired electron in non-bonding s-orbital Recent Gas Phase Studies Electronic Spectroscopy by CRDS [Lin], [Tonokura] Microwave Spectroscopy[Mc. Mahon] High Resolution IR Spectroscopy [Sharp] Matrix Isolation Studies [Friderichesen], [Radziszewski], [Pacansky], [Miller], [Engert], [Park] IR and UV Spectroscopy [Tonokura] 3

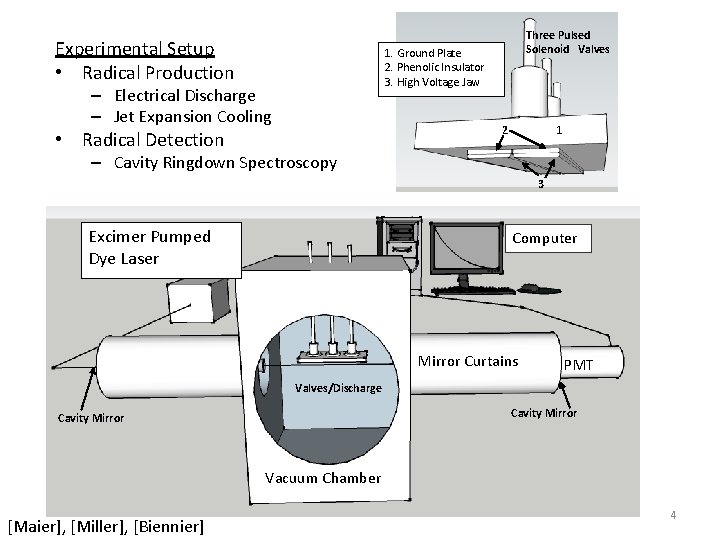
Experimental Setup • Radical Production Three Pulsed Solenoid Valves 1. Ground Plate 2. Phenolic Insulator 3. High Voltage Jaw – Electrical Discharge – Jet Expansion Cooling 2 • Radical Detection 1 – Cavity Ringdown Spectroscopy 3 Excimer Pumped Dye Laser Computer Mirror Curtains PMT Valves/Discharge Cavity Mirror Vacuum Chamber [Maier], [Miller], [Biennier] 4
![Cavity RingDown Spectroscopy Loss 2 altc2 L Total loss 1 Ral tcL Cavity Ring-Down Spectroscopy Loss = (2 al)(tc/2 L) Total loss = [(1 -R)+al] (tc/L)](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-5.jpg)
Cavity Ring-Down Spectroscopy Loss = (2 al)(tc/2 L) Total loss = [(1 -R)+al] (tc/L) a = 1. 16 x 10 -5 cm-1 l e-1 x 100 = 36. 8 t w/ abs= 12 ms Absorbing Sample Added R t empty= 18 ms R PMT Empty Cavity R R PMT ~ 5000 passes at 18 ms (path length from 0. 10 m to 500 m) 5

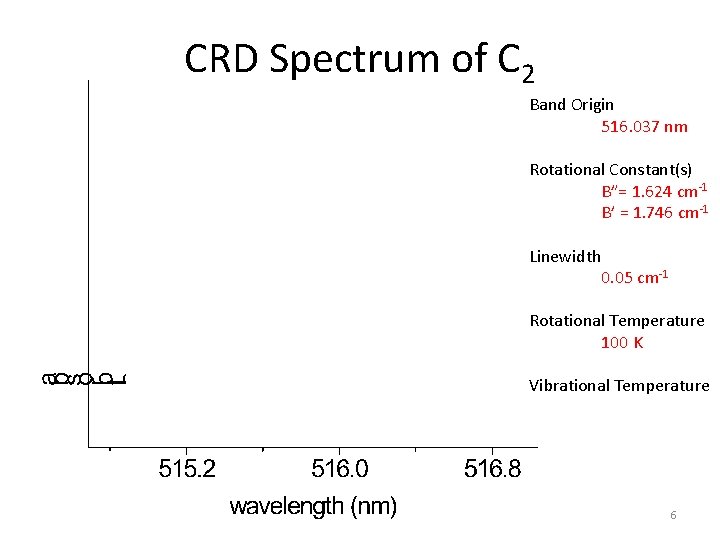
CRD Spectrum of C 2 PGopher Simulation Band Origin 516. 037 nm Rotational Constant(s) B”= 1. 624 cm-1 B’ = 1. 746 cm-1 Linewidth 0. 05 cm-1 Rotational Temperature 100 K Vibrational Temperature 6
![Long range scan Huang 7 Long range scan [Huang] 7](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-7.jpg)
Long range scan [Huang] 7

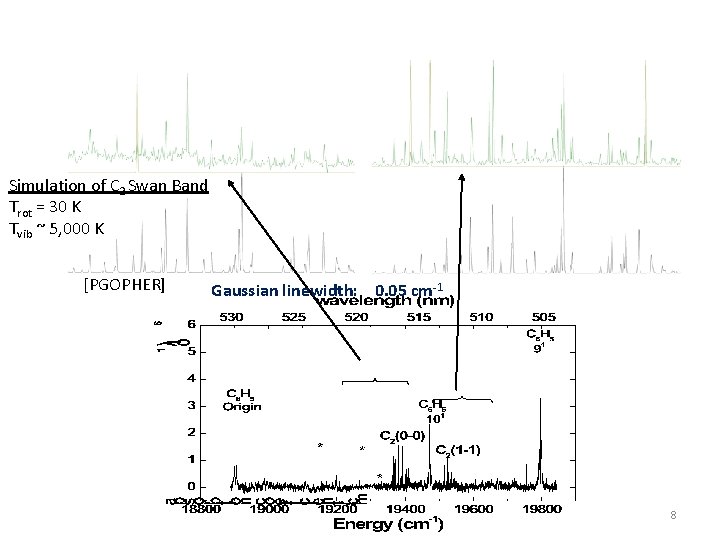
Simulation of C 2 Swan Band Trot = 30 K Tvib ~ 5, 000 K [PGOPHER] Gaussian linewidth: 0. 05 cm-1 8
![A 0 20947210 B 0 1867937 MW spec Mc Mahon C 0 09871498820 B A”: 0. 209472(10) B”: 0. 186793(7) MW spec [Mc. Mahon] C”: 0. 098714988(20) B](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-9.jpg)
A”: 0. 209472(10) B”: 0. 186793(7) MW spec [Mc. Mahon] C”: 0. 098714988(20) B 3 LYP/aug-cc-p. VDZ [Tonokura] A’: 0. 1964 B’: 0. 1846 C’: 0. 0952 12 B 1 -2 A 1 origin band Origin: 18901. 29(3) A: 0. 198(1) B: 0. 185(1) C: 0. 0957(5) Temp (K): 26. 6 Gaussian linewidth: 0. 05 cm-1 9

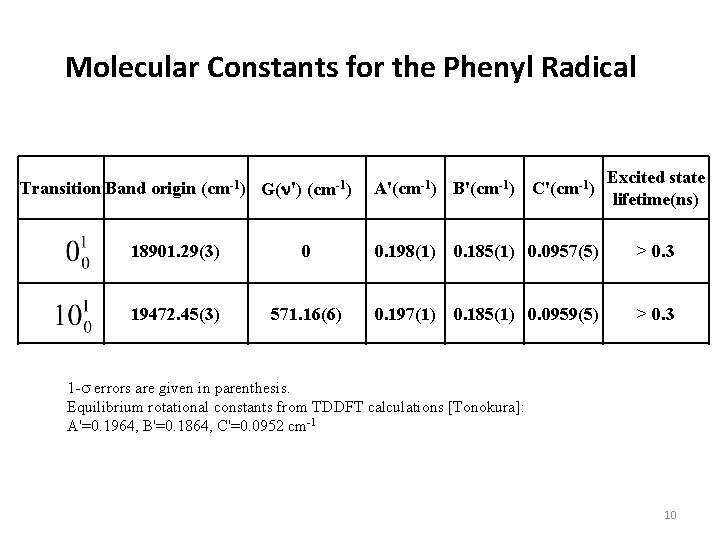
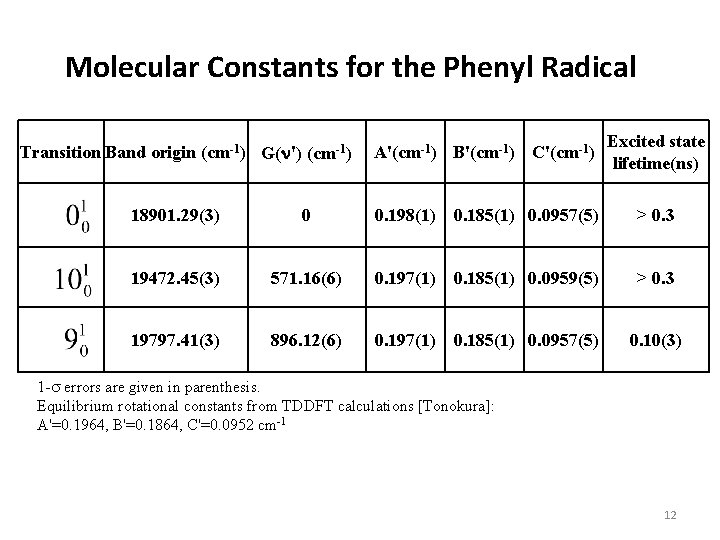
Molecular Constants for the Phenyl Radical Transition Band origin (cm-1) G(n') (cm-1) A'(cm-1) B'(cm-1) C'(cm-1) Excited state lifetime(ns) 18901. 29(3) 0 0. 198(1) 0. 185(1) 0. 0957(5) > 0. 3 19472. 45(3) 571. 16(6) 0. 197(1) 0. 185(1) 0. 0959(5) > 0. 3 19797. 41(3) 896. 12(6) 0. 197(1) 0. 185(1) 0. 0957(5) 0. 10(3) 1 -s errors are given in parenthesis. Equilibrium rotational constants from TDDFT calculations [Tonokura]: A'=0. 1964, B'=0. 1864, C'=0. 0952 cm-1 10

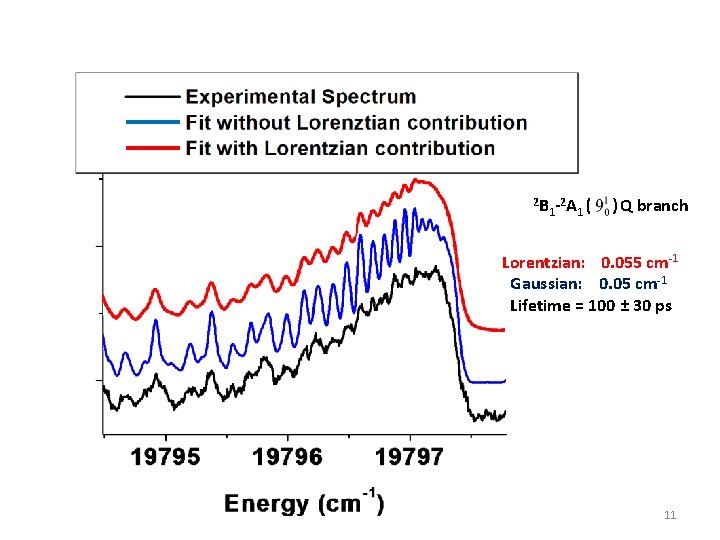
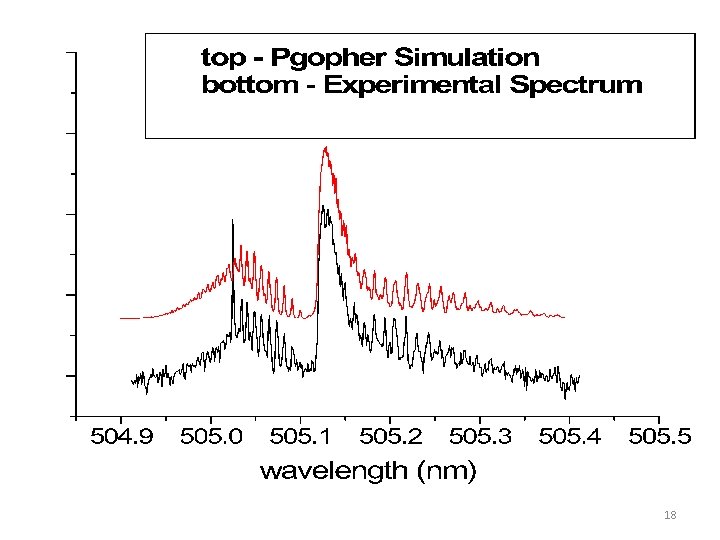
2 B -2 A ( 1 1 ) Q branch Lorentzian: 0. 055 cm-1 Gaussian: 0. 05 cm-1 Lifetime = 100 ± 30 ps 11

Molecular Constants for the Phenyl Radical Transition Band origin (cm-1) G(n') (cm-1) A'(cm-1) B'(cm-1) C'(cm-1) Excited state lifetime(ns) 18901. 29(3) 0 0. 198(1) 0. 185(1) 0. 0957(5) > 0. 3 19472. 45(3) 571. 16(6) 0. 197(1) 0. 185(1) 0. 0959(5) > 0. 3 19797. 41(3) 896. 12(6) 0. 197(1) 0. 185(1) 0. 0957(5) 0. 10(3) 1 -s errors are given in parenthesis. Equilibrium rotational constants from TDDFT calculations [Tonokura]: A'=0. 1964, B'=0. 1864, C'=0. 0952 cm-1 12
![12 B 1 X 2 A 1 Energy Transfer Oscillator Strength Kim 12 B 1 X 2 A 1 - Energy Transfer Oscillator Strength [Kim] =](https://slidetodoc.com/presentation_image_h2/ee8dc41c6a34b49cfeba30f1114cc080/image-13.jpg)
12 B 1 X 2 A 1 - Energy Transfer Oscillator Strength [Kim] = 0. 0016 trad = 2. 8 ms (CASSCF(7, 13)/6 -311+G**) For 12 B 1 (n 9 = 1) the lifetime was 100 ns Fluorescence quantum yield ~ 3. 4 x 10 -5 c 00 -1 m , 6 26 21 , 000 [Lin], [Negru] cm -1 13

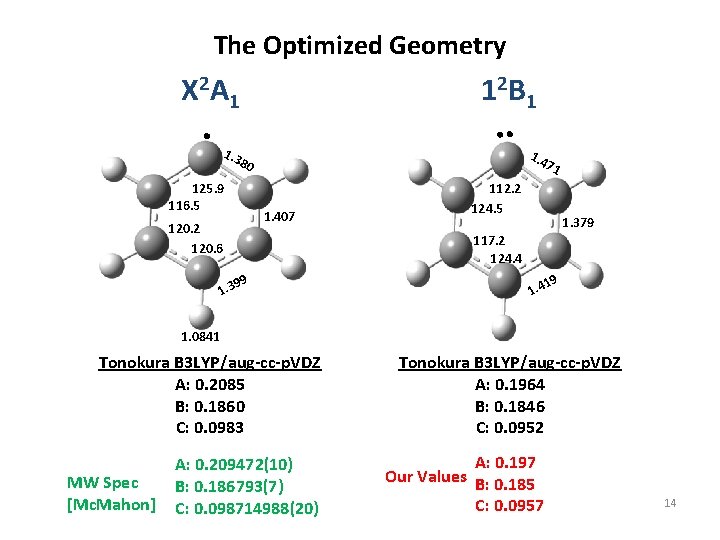
The Optimized Geometry X 2 A 1 1 2 B 1 1. 3 1. 4 80 125. 9 116. 5 1. 407 120. 2 120. 6 71 112. 2 124. 5 1. 379 117. 2 124. 4 99 1. 3 19 1. 4 1. 0841 Tonokura B 3 LYP/aug-cc-p. VDZ A: 0. 2085 B: 0. 1860 C: 0. 0983 MW Spec [Mc. Mahon] A: 0. 209472(10) B: 0. 186793(7) C: 0. 098714988(20) Tonokura B 3 LYP/aug-cc-p. VDZ A: 0. 1964 B: 0. 1846 C: 0. 0952 A: 0. 197 Our Values B: 0. 185 C: 0. 0957 14

Conclusions • Detection of discharge generated phenyl radical by CRDS in a jet expansion. • Measured G(n') and rotational constants for three fundamental modes. • Excited state lifetime is about 100 ps at n 9=1. • Rotational constants match best with constants from DFT calculation by Tonokura et al. 15

Thanks to: • Group Members and Colleagues: Dr Jeremy Merritt, Dr Humayun Kabir, Dr Beau Barker, Ivan Antonov, Dr Jiande Han, Kyle Mascaritolo, Luis Mendoza, Dr Shucheng Xu • Cody Anderson at the Emory Machine Shop • Thank you for listening! 16

References • • • Porter G. ; Ward B. Proc. R. Soc. London, Ser. A 1965, 287, 457. J. E. Bennett, B. Mile, A. Thomas, Chem. Comm. London (1965) 265. J. E. Bennett, B. Mile, A. Thomas, Proc. Royal Soc. London, Series A: Mathemat. Phys. Eng. Sci. 293 (1966) 246. P. H. Kasai, E. Hedaya, E. B. Whipple, J. Am. Chem. Soc. 91 (1969) 4364. J. Pacansky, J. Bargon, J. Am. Chem. Soc. 97 (1975) 6896. J. H. Miller, L. Andrews, P. A. Lund, P. N. Schatz, J. Chem. Phys. 73 (1980) 4932. J. G. Radziszewski, Chem. Phys. Lett. 301 (1999) 565. J. G. Radziszewski, M. Gil, A. Gorski, J. Spanget-Larsen, J. Waluk, B. J. Mroz, J. Chem. Phys. 115 (2001) 9733. J. M. Engert, B. Dick, Appl. Phys. B Lasers Opt. 63 (1996) 531. A. V. Friderichsen, J. G. Radziszewski, M. R. Nimlos, P. R. Winter, D. C. Dayton, D. E. David, G. B. Ellison, J. Am. Chem. Soc. 123 (2001) 1977. J. Park, S. Burova, A. S. Rodgers, M. C. Lin, Chem. Phys. Process Combust. (1999) 308. 17

18

19

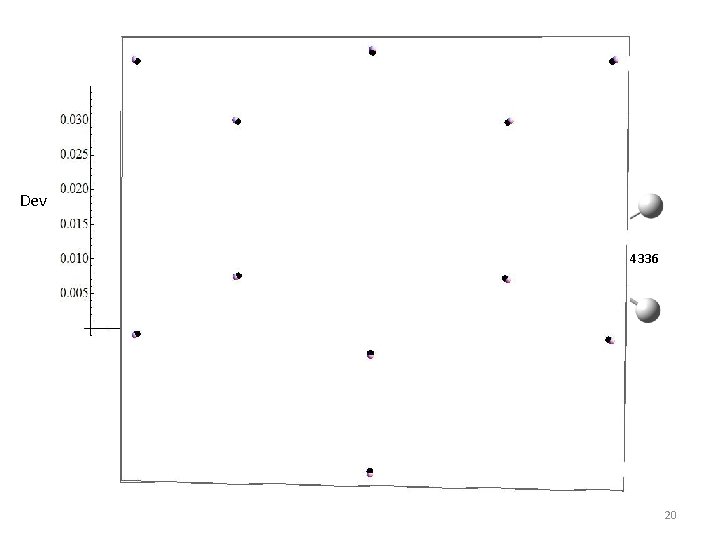
A: 0. 1976 B: 0. 1852 C: 0. 0951 Extra Geometry From: Dev = d. A+d. B+d. C 2 B 1. 0 1(v’=9) 84 Dev 1. 4 3 04 6 126. 0 116. 5 851 1. 0 1. 4336 120. 2 120. 6 260 1. 4 Factor 1. 0841 Ring Growth A: 0. 2016 B: 0. 1801 C: 0. 0951 20

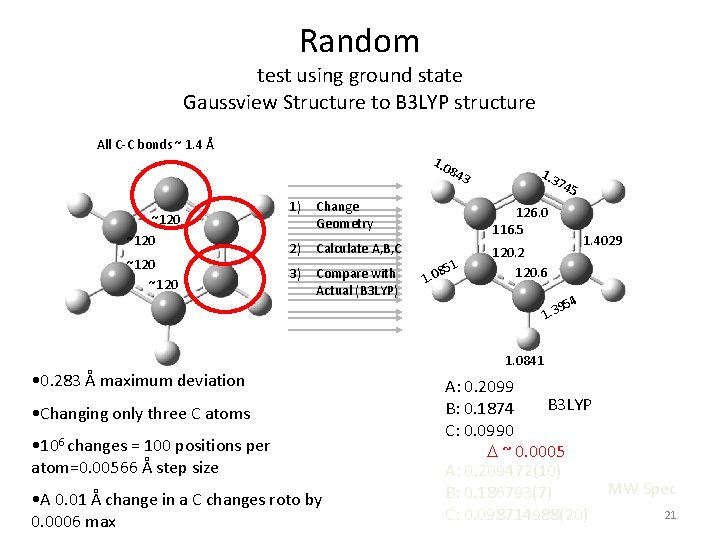
Random test using ground state Gaussview Structure to B 3 LYP structure All C-C bonds ~ 1. 4 Å 1. 0 84 ~120 3 1) Change Geometry 126. 0 116. 5 2) Calculate A, B, C 3) Compare with Actual (B 3 LYP) 120. 2 120. 6 51 8 1. 0 74 5 1. 4029 54 9 1. 3 1. 0841 • 0. 283 Å maximum deviation • Changing only three C atoms • 106 changes = 100 positions per atom=0. 00566 Å step size • A 0. 01 Å change in a C changes roto by 0. 0006 max A: 0. 2099 B 3 LYP B: 0. 1874 C: 0. 0990 D ~ 0. 0005 A: 0. 209472(10) MW Spec B: 0. 186793(7) 21 C: 0. 098714988(20)

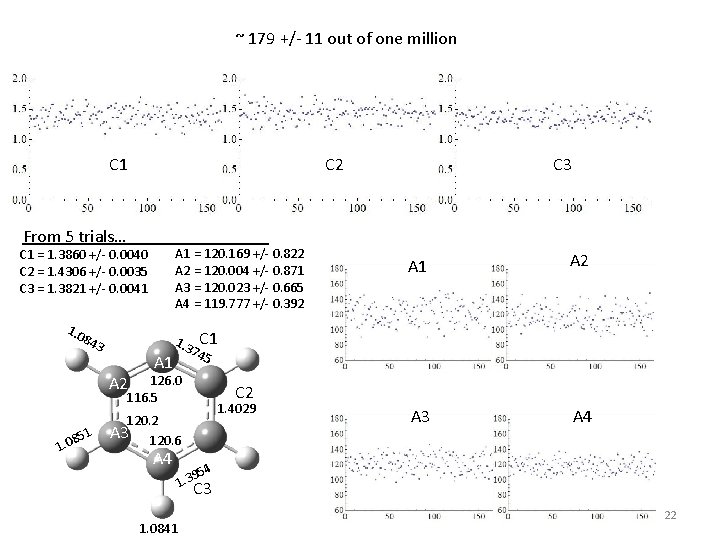
~ 179 +/- 11 out of one million C 1 C 2 From 5 trials… . A 1 = 120. 169 +/- 0. 822 A 2 = 120. 004 +/- 0. 871 A 3 = 120. 023 +/- 0. 665 A 4 = 119. 777 +/- 0. 392 C 1 = 1. 3860 +/- 0. 0040 C 2 = 1. 4306 +/- 0. 0035 C 3 = 1. 3821 +/- 0. 0041 1. 0 84 3 A 1 1. 3 51 8 1. 0 A 2 A 3 A 4 C 1 5 C 2 1. 4029 120. 2 A 3 120. 6 A 4 A 1 74 126. 0 116. 5 A 2 C 3 95 1. 3 4 C 3 1. 0841 22

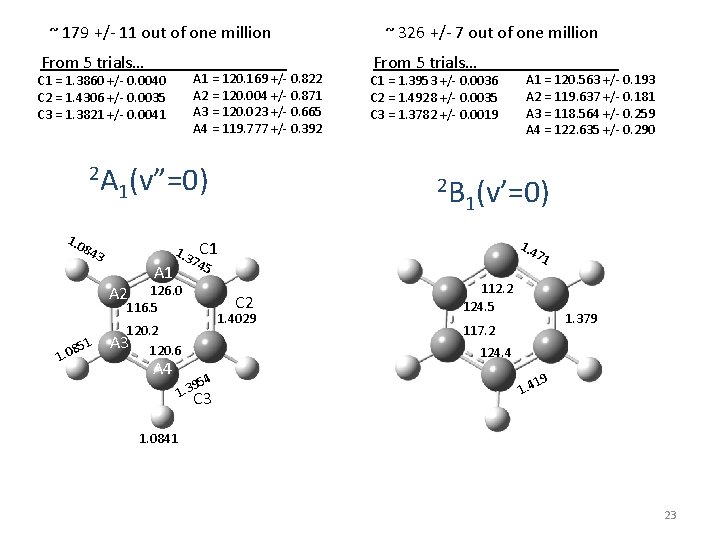
~ 179 +/- 11 out of one million From 5 trials… . A 1 = 120. 169 +/- 0. 822 A 2 = 120. 004 +/- 0. 871 A 3 = 120. 023 +/- 0. 665 A 4 = 119. 777 +/- 0. 392 C 1 = 1. 3860 +/- 0. 0040 C 2 = 1. 4306 +/- 0. 0035 C 3 = 1. 3821 +/- 0. 0041 2 A 1. 0 1(v”=0) 84 3 A 1 1. 3 51 8 1. 0 C 1 = 1. 3953 +/- 0. 0036 C 2 = 1. 4928 +/- 0. 0035 C 3 = 1. 3782 +/- 0. 0019 2 B . A 1 = 120. 563 +/- 0. 193 A 2 = 119. 637 +/- 0. 181 A 3 = 118. 564 +/- 0. 259 A 4 = 122. 635 +/- 0. 290 1(v’=0) 1. 4 74 5 C 2 1. 4029 120. 2 A 3 120. 6 A 4 From 5 trials… C 1 126. 0 116. 5 A 2 ~ 326 +/- 7 out of one million 71 112. 2 124. 5 1. 379 117. 2 124. 4 95 1. 3 4 C 3 19 1. 4 1. 0841 23