Caution The PHIL device is not clearedapproved by


Caution: The PHIL™ device is not cleared/approved by the U. S. FDA for sale or use in the United States.

PHIL™ Composition • PHIL™ is a liquid embolic agent made of a co-polymer dissolved in DMSO during the liquid phase and linked with an iodine agent for radiopacity purpose during injection Component 1 – Non-adhesive co-polymer-based liquid embolic material • Hydroxyethyl methacrylate (PHEMA) – Radiopacity from Iodine contrast agent covalently bonded Component 2 – Dissolved in DMSO solvent • Dimethyl Sulfoxide

Indications For Use (CE mark) The PHIL device is intended for use in the embolization of lesions in the peripheral and neurovasculature, including arteriovenous malformations and hypervascular tumors
PHIL™ Copolymer Main Characteristics • PHIL™ copolymer – Soluble in DMSO – Will solidify in an aqueous (Water) environment – Non-Thrombogenic • Shown per Pre-clinical testing – Non-exothermic • No chemical reaction as n. BCA polymerization – Non-adhesive – Radiopaque – Cohesive
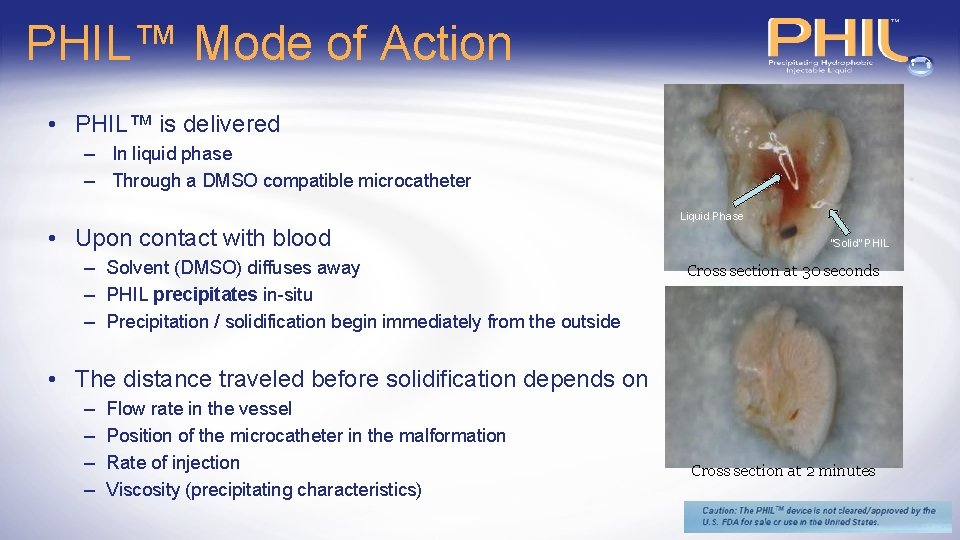
PHIL™ Mode of Action • PHIL™ is delivered – In liquid phase – Through a DMSO compatible microcatheter Liquid Phase • Upon contact with blood – Solvent (DMSO) diffuses away – PHIL precipitates in-situ – Precipitation / solidification begin immediately from the outside “Solid” PHIL Cross section at 30 seconds • The distance traveled before solidification depends on – – Flow rate in the vessel Position of the microcatheter in the malformation Rate of injection Viscosity (precipitating characteristics) Cross section at 2 minutes

PHIL™ System Components • 1 cc of PHIL in pre-filled Sterile syringe • 1 cc of DMSO in pre-filled Sterile syringe • Catheter specific adapters ü 3 separate sterile pouches IFU
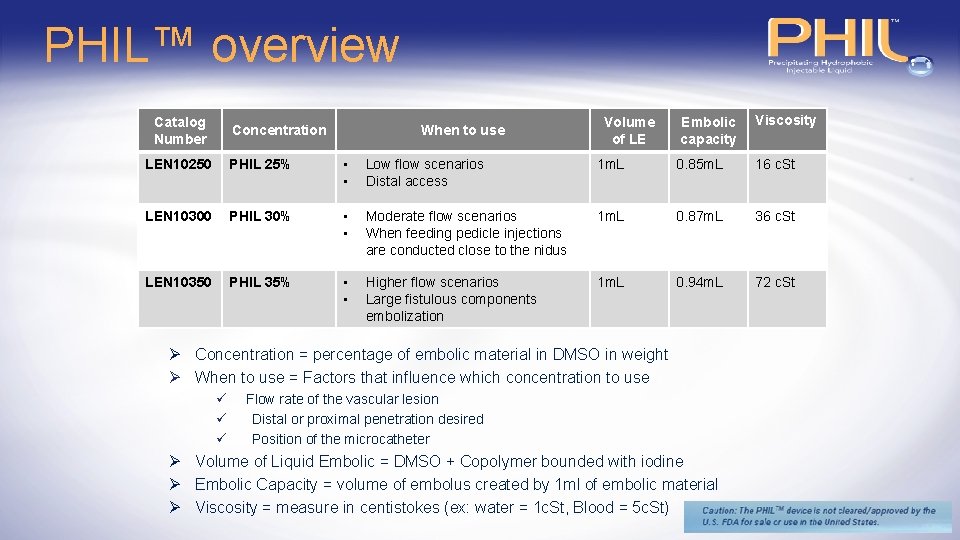
PHIL™ overview Catalog Number Concentration When to use Volume of LE Embolic capacity Viscosity LEN 10250 PHIL 25% • • Low flow scenarios Distal access 1 m. L 0. 85 m. L 16 c. St LEN 10300 PHIL 30% • • Moderate flow scenarios When feeding pedicle injections are conducted close to the nidus 1 m. L 0. 87 m. L 36 c. St LEN 10350 PHIL 35% • • Higher flow scenarios Large fistulous components embolization 1 m. L 0. 94 m. L 72 c. St Ø Concentration = percentage of embolic material in DMSO in weight Ø When to use = Factors that influence which concentration to use ü ü ü Flow rate of the vascular lesion Distal or proximal penetration desired Position of the microcatheter Ø Volume of Liquid Embolic = DMSO + Copolymer bounded with iodine Ø Embolic Capacity = volume of embolus created by 1 ml of embolic material Ø Viscosity = measure in centistokes (ex: water = 1 c. St, Blood = 5 c. St)

Key Features and benefits • Ready to use – No Shaking – Pre-filled syringes – Sterile set • Optimized visibility – Perfect homogeneity of radiopacity of Liquid embolic – Optimum visibility all along the procedure – Visibility of Microcatheter tip during the treatment • No metallic component – Minimize (streak) artefact during control imagery – No saturated radio-opaque cast to facilitate stages endovascular treatment – Compatible with surgical resection – No tattoo effect of the Tantalum powder in superficial malformations treatment • High embolic Capacity – Less DMSO injected – More Embolus created with 1 m. L of Embolic material
- Slides: 9