Catalysts speed up the rate of the reaction
- Slides: 14

Catalysts §speed up the rate of the reaction. §lowers the energy input required for a chemical reaction to happen §remains unchanged at the end of the reaction skool

Enzymes § Enzymes are Biological catalysts §Enzymes control chemical reactions that take place in the cytoplasm. §http: //programs. northlandcollege. edu/biology/Biology 1111/animations/enz yme. html §Catalase in an example of an enzyme made by living cells

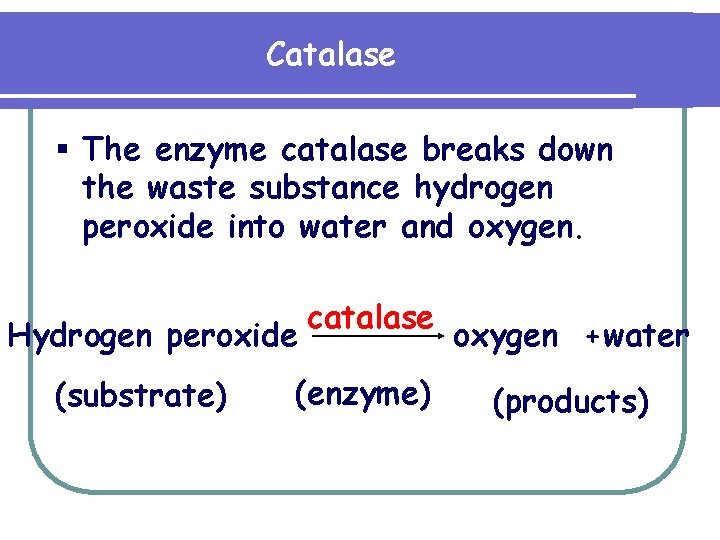
Catalase § The enzyme catalase breaks down the waste substance hydrogen peroxide into water and oxygen. Hydrogen peroxide (substrate) catalase (enzyme) oxygen +water (products)

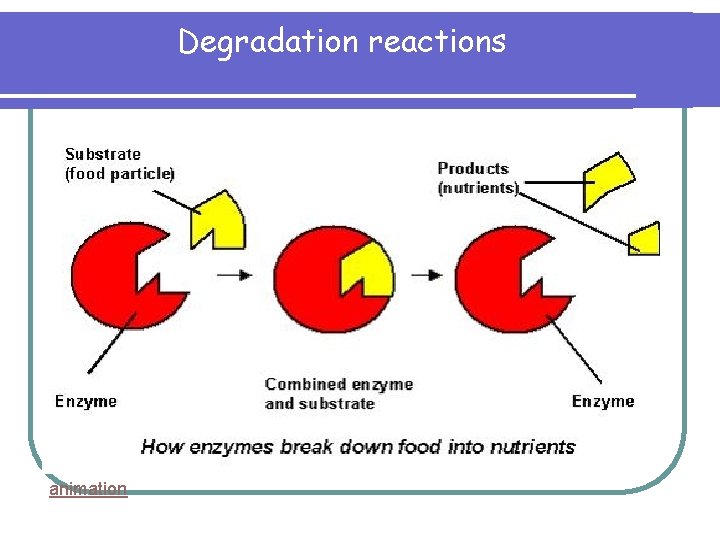
Degradation reactions animation

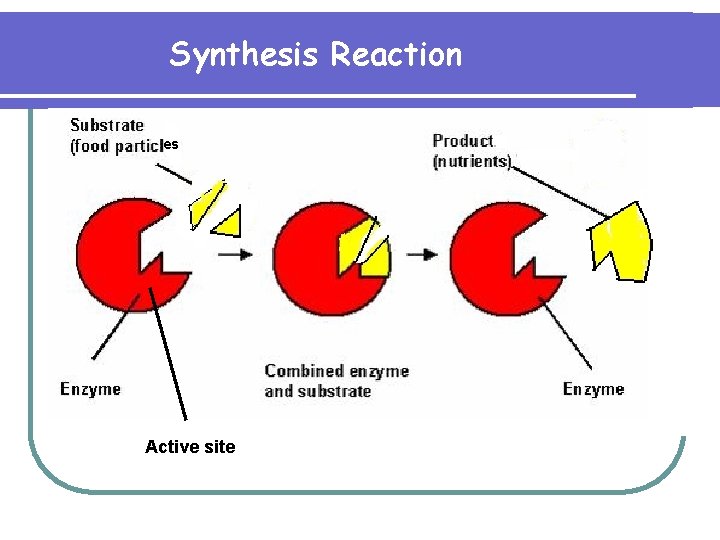
Synthesis Reaction es Active site

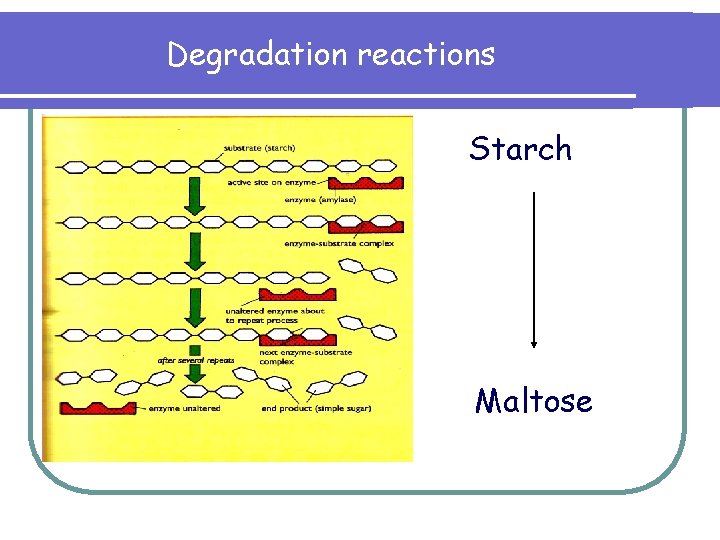
Degradation reactions Starch Maltose

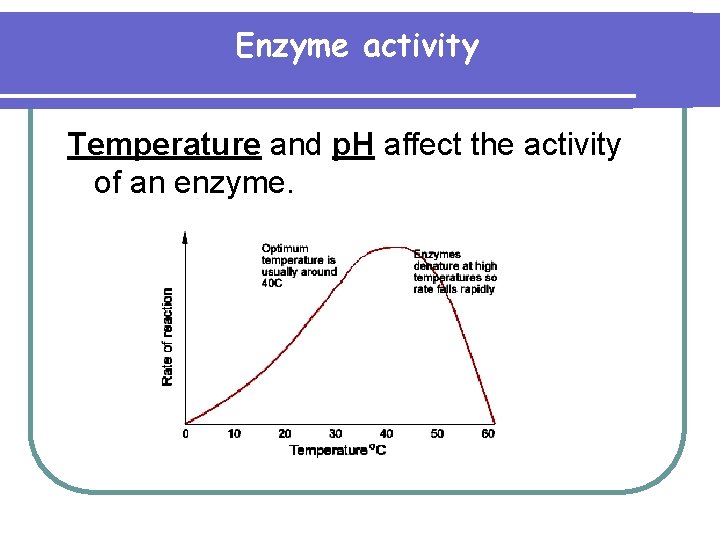
Enzyme activity Temperature and p. H affect the activity of an enzyme.

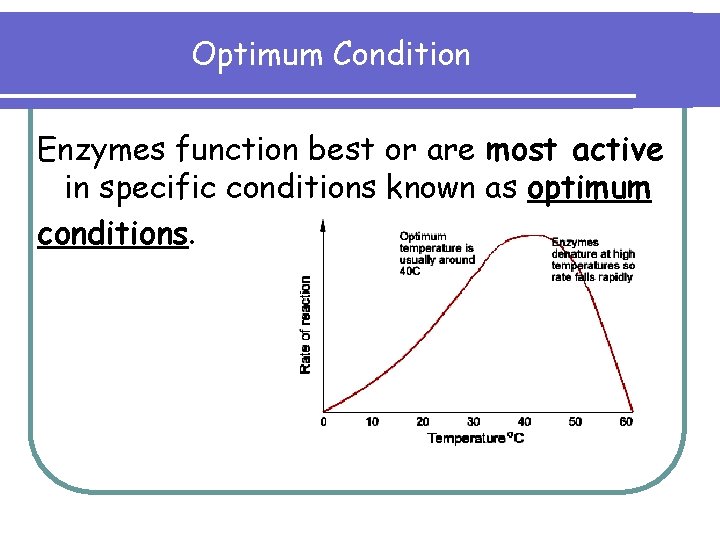
Optimum Condition Enzymes function best or are most active in specific conditions known as optimum conditions.

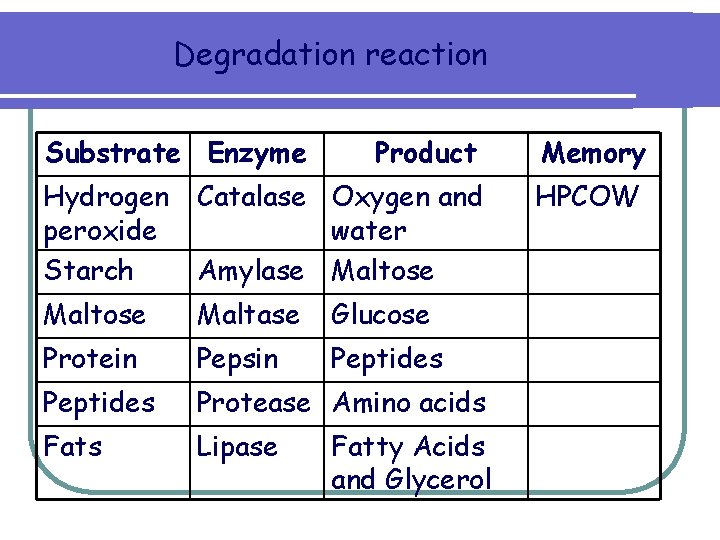
Degradation reaction Substrate Enzyme Product Memory Hydrogen peroxide Starch Catalase Oxygen and water Amylase Maltose HPCOW Maltose Maltase Glucose Protein Pepsin Peptides Protease Amino acids Fats Lipase Fatty Acids and Glycerol

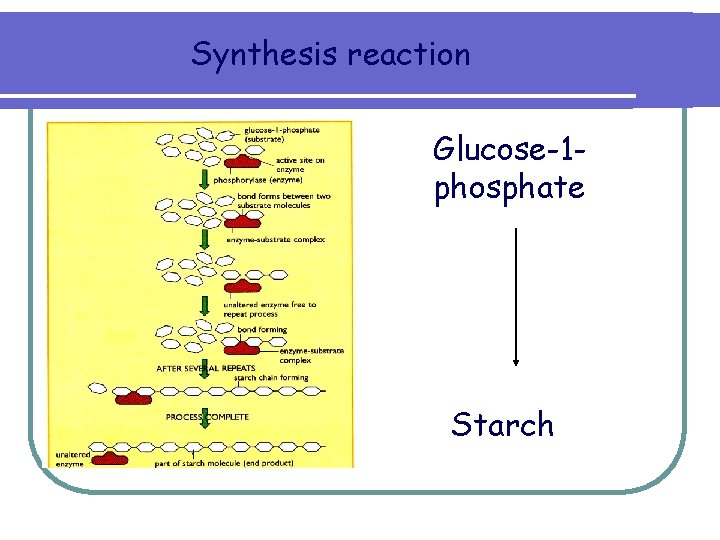
Synthesis reaction Glucose-1 phosphate Starch

Properties of enzymes Speed up reactions. Made of protein. Are specific. Not used up during the reaction. Require optimum conditions at which they work best. At high temperature they become denatured

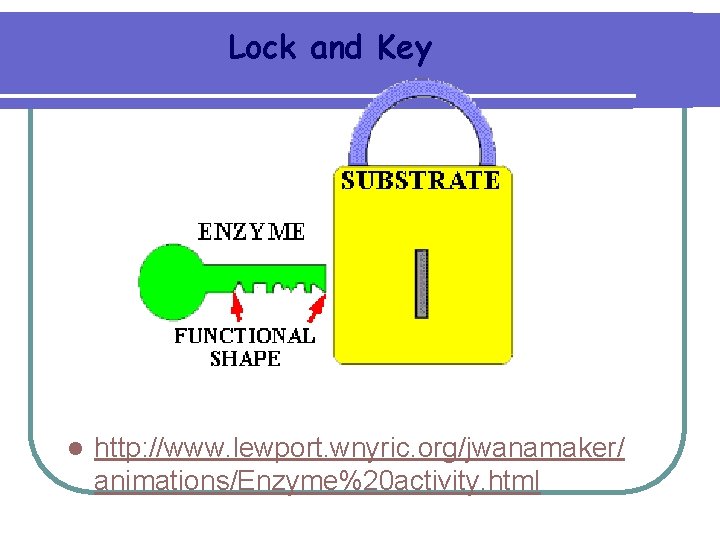
Lock and Key l http: //www. lewport. wnyric. org/jwanamaker/ animations/Enzyme%20 activity. html

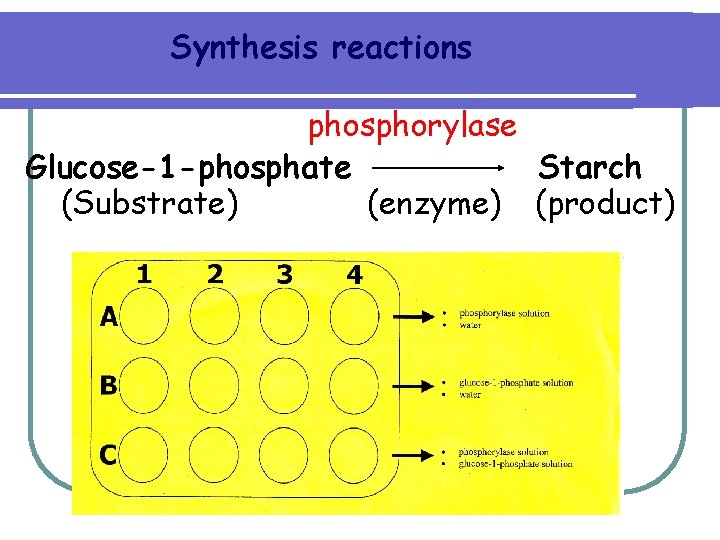
Synthesis reactions phosphorylase Glucose-1 -phosphate Starch (Substrate) (enzyme) (product)

This powerpoint was kindly donated to www. worldofteaching. com http: //www. worldofteaching. com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching.