Catalyst Create and fill in the following table
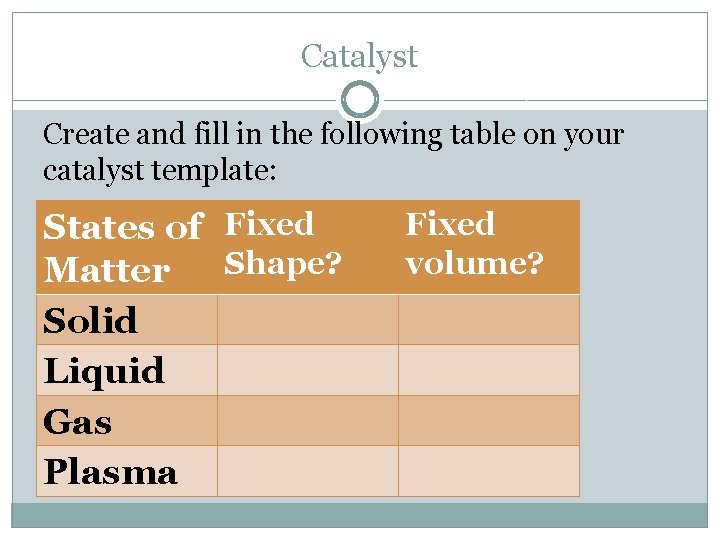
Catalyst Create and fill in the following table on your catalyst template: States of Fixed Shape? Matter Solid Liquid Gas Plasma Fixed volume?

Today’s Objectives �SWBAT describe the arrangement of particles in each state of matter. �SWBAT describe the motion of particles in each state of matter. �SWBAT describe the speed of particles in each state of matter. �SBWAT demonstrate what happens to particles of a substance when heated or cooled. SPI 0807. 9. 6: Compare the particle arrangement and type of particle motion associated with different states of matter.

What will happen to the food coloring?

Today’s title… Particle Arrangement!
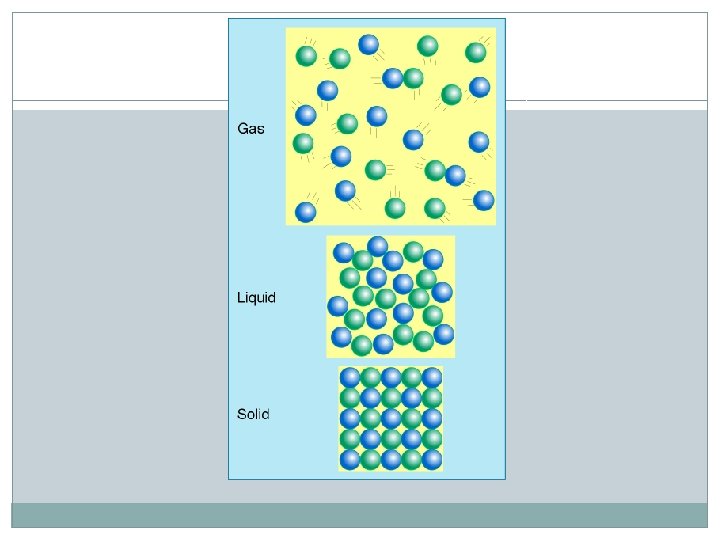
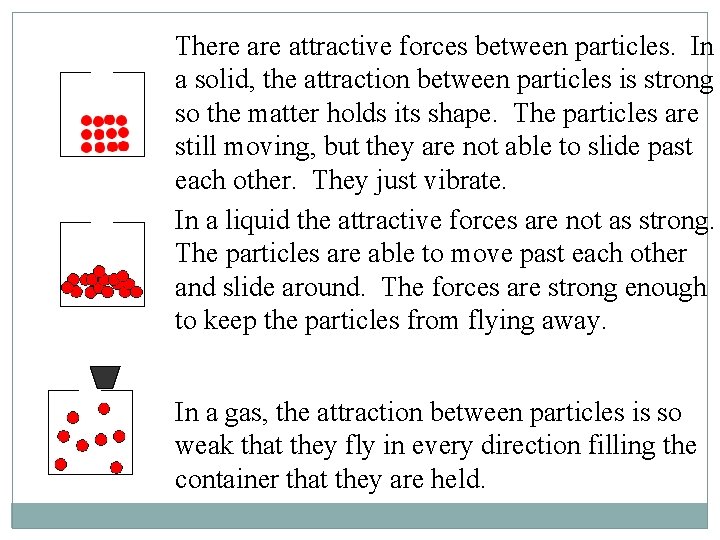
There attractive forces between particles. In a solid, the attraction between particles is strong so the matter holds its shape. The particles are still moving, but they are not able to slide past each other. They just vibrate. In a liquid the attractive forces are not as strong. The particles are able to move past each other and slide around. The forces are strong enough to keep the particles from flying away. In a gas, the attraction between particles is so weak that they fly in every direction filling the container that they are held.

Solids-Particle Speed and Motion �Vibrate in place �Moving the slowest of all states of matter
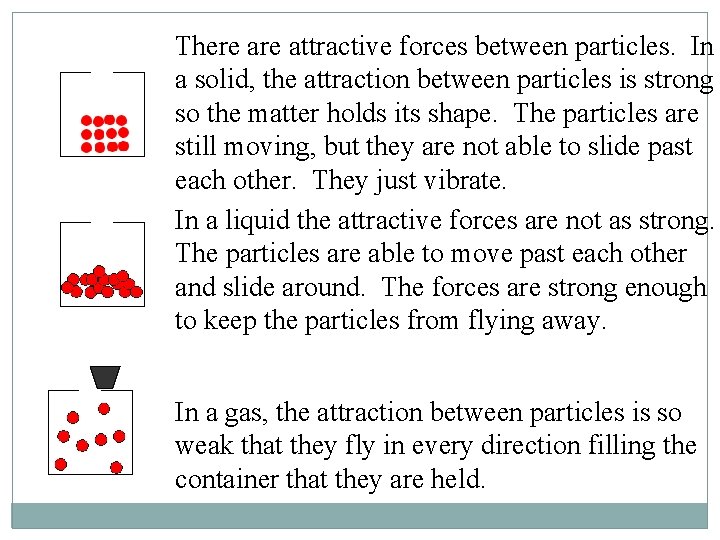
There attractive forces between particles. In a solid, the attraction between particles is strong so the matter holds its shape. The particles are still moving, but they are not able to slide past each other. They just vibrate. In a liquid the attractive forces are not as strong. The particles are able to move past each other and slide around. The forces are strong enough to keep the particles from flying away. In a gas, the attraction between particles is so weak that they fly in every direction filling the container that they are held.

Liquids-Particle Speed and Motion �Move faster than a solid but slower than a gas �Flow (they are fluid)
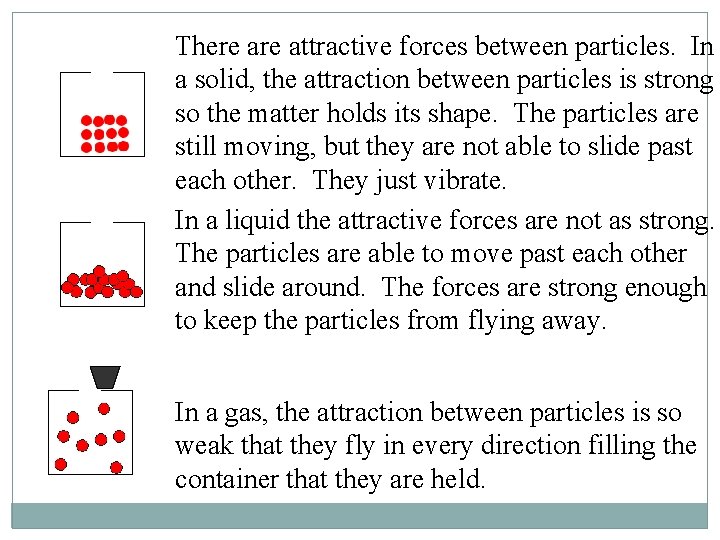
There attractive forces between particles. In a solid, the attraction between particles is strong so the matter holds its shape. The particles are still moving, but they are not able to slide past each other. They just vibrate. In a liquid the attractive forces are not as strong. The particles are able to move past each other and slide around. The forces are strong enough to keep the particles from flying away. In a gas, the attraction between particles is so weak that they fly in every direction filling the container that they are held.

Gases-Particle Speed and Motion �Move the very rapidly in every which direction

Ranking Speed of Particles �Speed of particles: Gas> Liquid> Solid �Plasma particles are even faster than the three states of matter
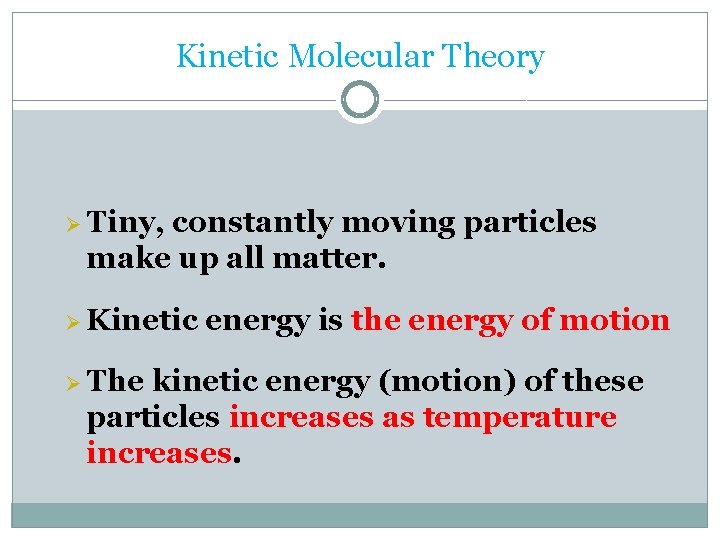
Kinetic Molecular Theory Ø Tiny, constantly moving particles make up all matter. Ø Kinetic Ø The energy is the energy of motion kinetic energy (motion) of these particles increases as temperature increases.

�When Frosty the Snowman melts, what happens to the distance between his particles? �What happens to the speed and motion of those particles?

�Iodine solid will turn directly into vapor �What is the name of this phase change? �What happens to the distance between the particles when this occurs? �What happens to the speed and motion of the particles when this phase change occurs?
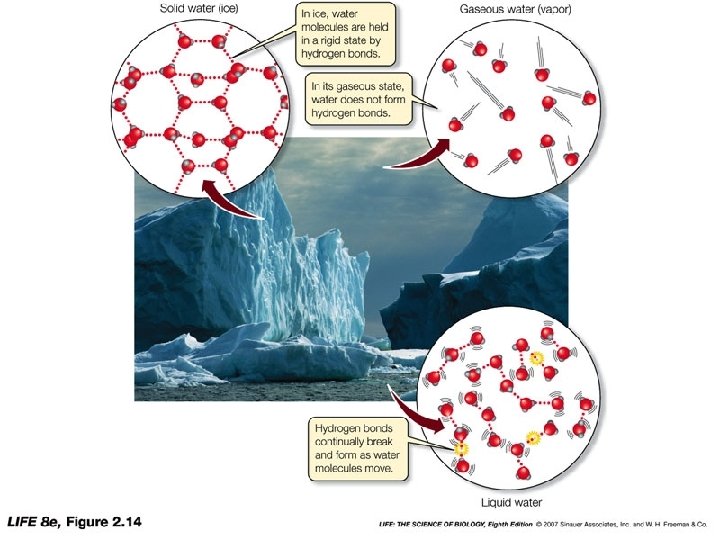

SOLIDS: Fixed shape, fixed volume, high density, particles extremely close together, vibrate, low energy Solid H 2 O: Below 0°C

LIQUID: No fixed shape, fixed volume, particles flow, takes shape of container Liquid H 2 O: Between 0 -100°C

GAS: No fixed shape, no fixed volume, particles flow, LARGE distance between particles, particles move RAPIDLY in every direction Gaseous H 2 O: Above 100°C
Particle Interactive �http: //phet. colorado. edu/en/simulation/states-of- matter-basics
- Slides: 20