Catalyst 1 What is the difference between an

Catalyst 1. What is the difference between an ionic and covalent bond? 2. Name Na 2 O. 3. Name NO 3. 4. How many grams are in 4 moles of CH 4? 5. Balance and classify the following reaction: Mg + O 2 Mg. O

Let’s come together A SYNTHESIS (COMPOSITION) REACTION is two reactants come together to form one compound General: A + B AB Example: 2 Na + Cl 2 2 Na. Cl +

Practice �Predict the products of a synthesis reaction. What are the reactants? �Al + O 2 ? What are the products? �It’s a synthesis reaction, so we know Al and O are going to “hook up” to make a compound…. �You need to KRISS KROSS to figure out the correct compound that was made! Al (s) + O 2 (g) 3+ 2 Al Al. O O 3 2 O

I don’t think this is working out… A DECOMPOSITION REACTION is one in which one reactant breaks apart into two or more products General: AB A + B Example: Ca. CO 3 Ca. O + CO 2 +
�A SINGLE-REPLACEMENT REACTION is when one element or ion is swapped. �There always 2 reactants and two products. �One reactant is alone and a different element is alone in the products. �General: A + BX AX + B �Example: KI + Cl 2 KCl + I 2
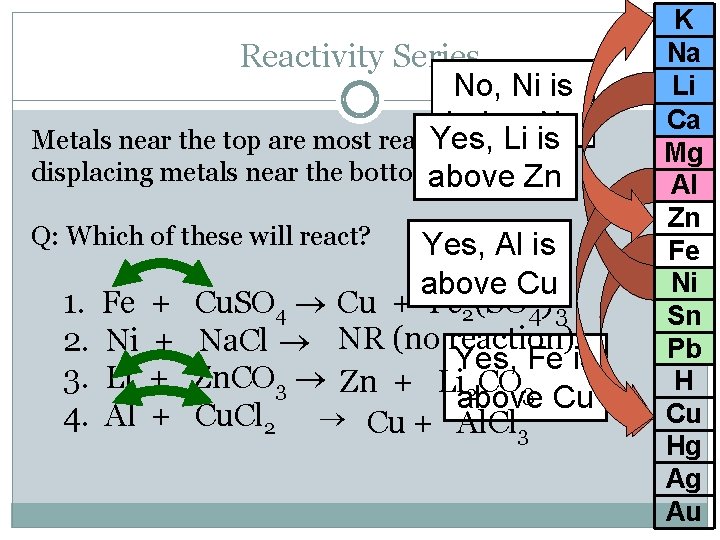
Reactivity Series No, Ni is below Na Yes, and Li will is Metals near the top are most reactive displacing metals near the bottom. above Zn Q: Which of these will react? 1. 2. 3. 4. Fe Ni Li Al + + Yes, Al is above Cu Cu. SO 4 Cu + Fe 2(SO 4)3 Na. Cl NR (no reaction) Yes, Fe is Zn. CO 3 Zn + Li 2 CO 3 above Cu Cu. Cl 2 Cu + Al. Cl 3 K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au
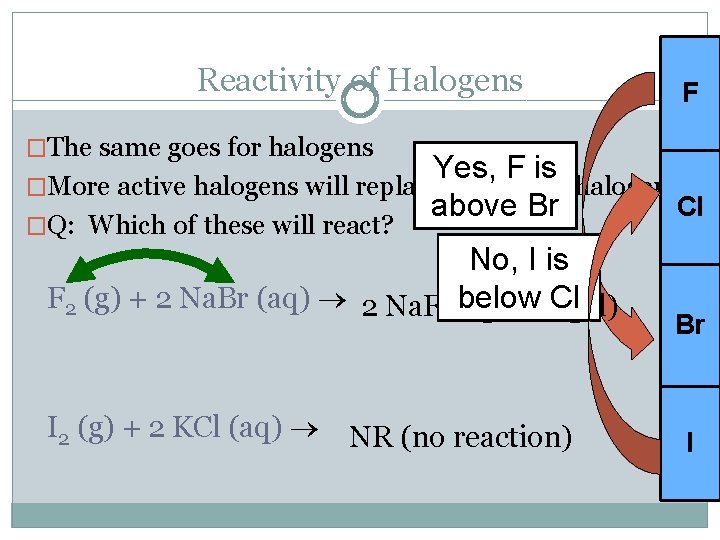
Reactivity of Halogens F �The same goes for halogens Yes, F is �More active halogens will replace less active halogens Cl above Br �Q: Which of these will react? No, I is F 2 (g) + 2 Na. Br (aq) 2 Na. F (aq) below Cl 2 (l) + Br I 2 (g) + 2 KCl (aq) NR (no reaction) Br I
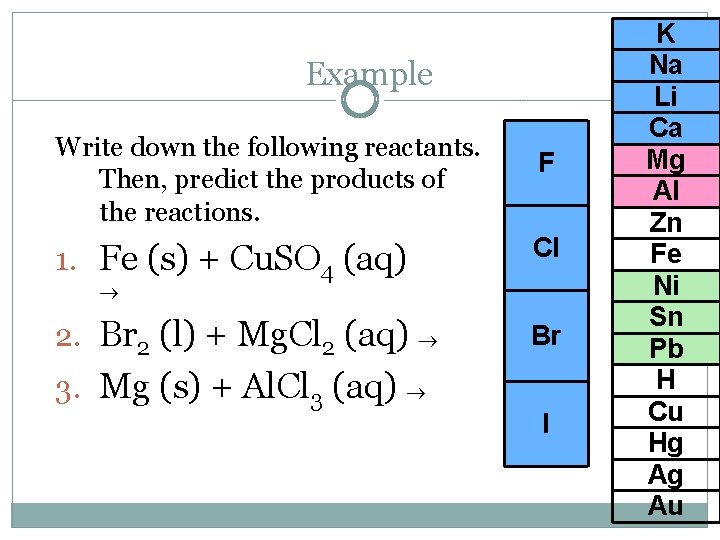
Example Write down the following reactants. Then, predict the products of the reactions. F 1. Fe (s) + Cu. SO 4 (aq) Cl 2. Br 2 (l) + Mg. Cl 2 (aq) Br 3. Mg (s) + Al. Cl 3 (aq) I K Na Li Ca Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au

Swing Fever! A DOUBLE-REPLACEMENT REACTION is one in which ions switch places. You must KRISS -KROSS to write ALL formulas! �General: AX + BY AY + BX �Example: K 2 CO 3 + Cu. SO 4 Cu. CO 3+ K 2 SO 4

Let’s think about the positive and the negative…cations and anions AX + BY AY + BX A and B are cations (positively charged) X and Y are anions (negatively charged) You can see the anions have switched places and are now bonded to the other cations in the reaction

DON’T FORGET! For which reactions do I need to use Kriss Kross? �Synthesis �Single Replacement �Double Replacement Which elements are diatomic when they are by themselves (have a subscript of two)? �Hydrogen (H 2) �Nitrogen (N 2) �Oxygen (O 2) �Chlorine (Cl 2) �Fluorine (F 2) �Bromine (Br 2) �Iodine (I 2)

It’s getting hot in here… � A COMBUSTION REACTION is � � when any reactant with C and H in it reacts with O 2 to produce CO 2 and H 2 O A hydrocarbon and oxygen are always the reactants Carbon dioxide and water are always the products! General: Cx. Hx + O 2 CO 2 + H 2 O Example: C 3 H 8 + O 2 CO 2 + H 2 O
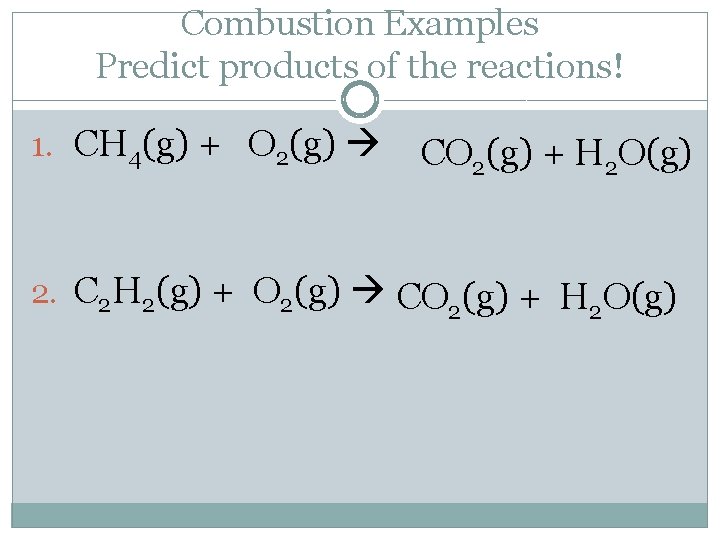
Combustion Examples Predict products of the reactions! 1. CH 4(g) + O 2(g) CO 2(g) + H 2 O(g) 2. C 2 H 2(g) + O 2(g) CO (g) + H O(g) 2 2
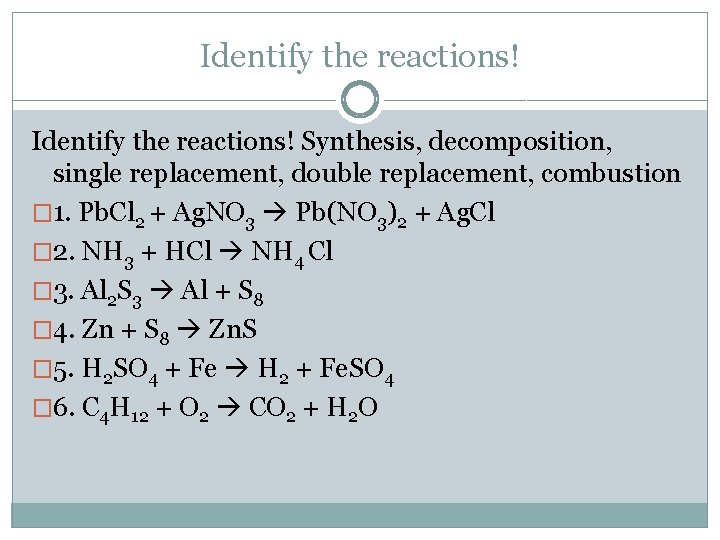
Identify the reactions! Synthesis, decomposition, single replacement, double replacement, combustion � 1. Pb. Cl 2 + Ag. NO 3 Pb(NO 3)2 + Ag. Cl � 2. NH 3 + HCl NH 4 Cl � 3. Al 2 S 3 Al + S 8 � 4. Zn + S 8 Zn. S � 5. H 2 SO 4 + Fe H 2 + Fe. SO 4 � 6. C 4 H 12 + O 2 CO 2 + H 2 O
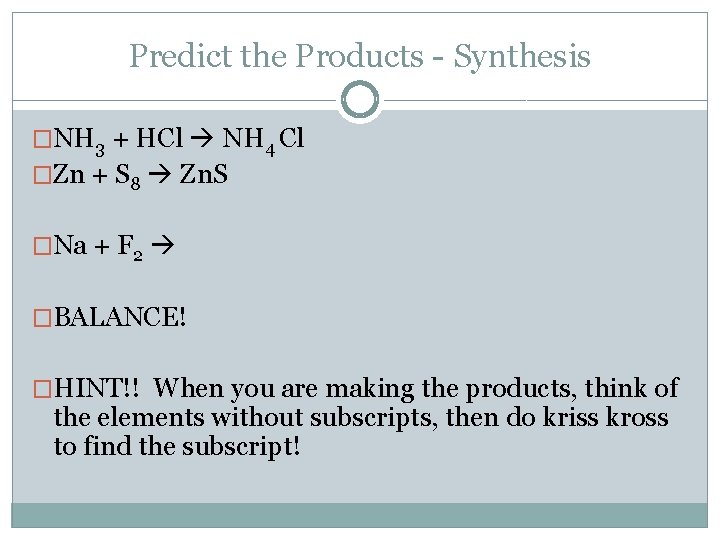
Predict the Products - Synthesis �NH 3 + HCl NH 4 Cl �Zn + S 8 Zn. S �Na + F 2 �BALANCE! �HINT!! When you are making the products, think of the elements without subscripts, then do kriss kross to find the subscript!
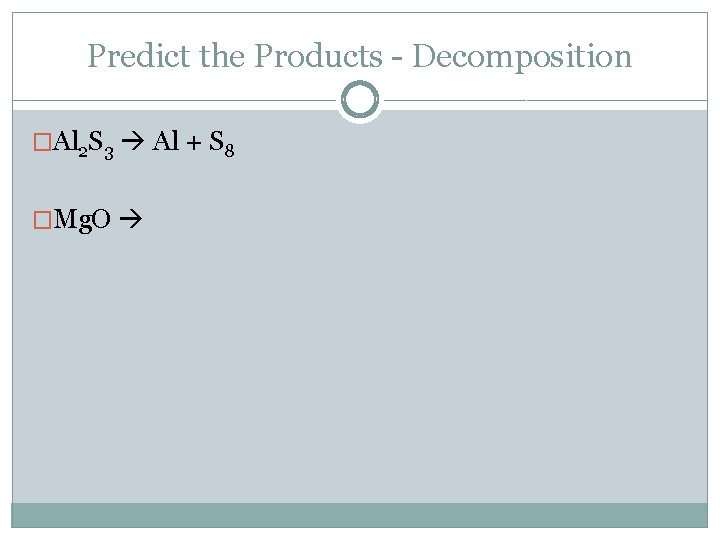
Predict the Products - Decomposition �Al 2 S 3 Al + S 8 �Mg. O
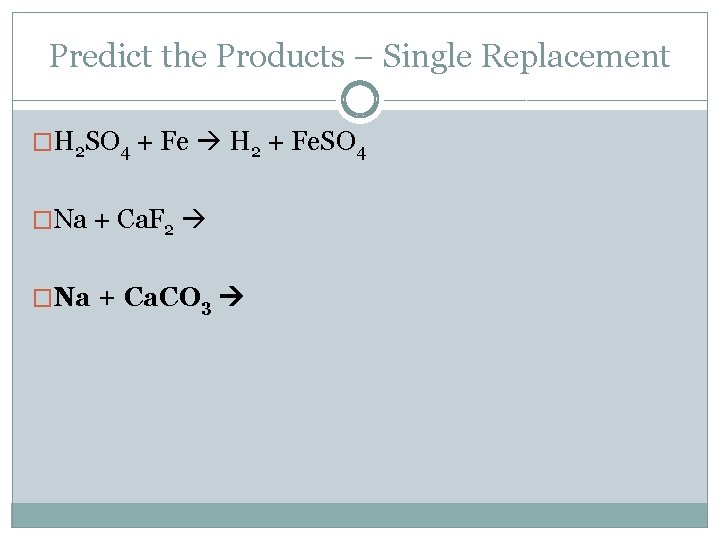
Predict the Products – Single Replacement �H 2 SO 4 + Fe H 2 + Fe. SO 4 �Na + Ca. F 2 �Na + Ca. CO 3

Predict the Products - double replacement � Pb. Cl 2+ Ag. NO 3 Pb(NO 3)2 + Ag. Cl �Na. OH + Cu. SO 4 �Li 2 SO 4 + Mg. Cl 2
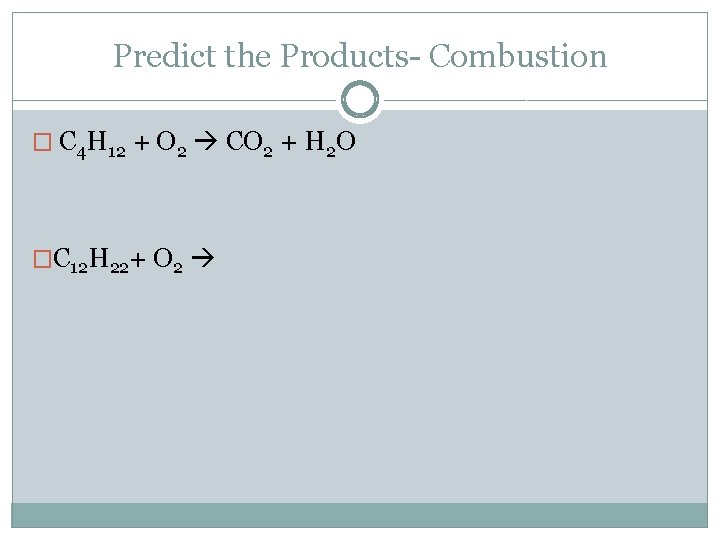
Predict the Products- Combustion � C 4 H 12 + O 2 CO 2 + H 2 O �C 12 H 22+ O 2

Don’t Forget!! �Kriss-kross changes to find your subscripts in a synthesis (combination, composition), single replacement, or double replacement reaction � 1 mol = 6. 022 x 1023 atoms, molecules, particles � 1 mol = 22. 4 L �The only way you can convert between different substances is by converting to the mole first!
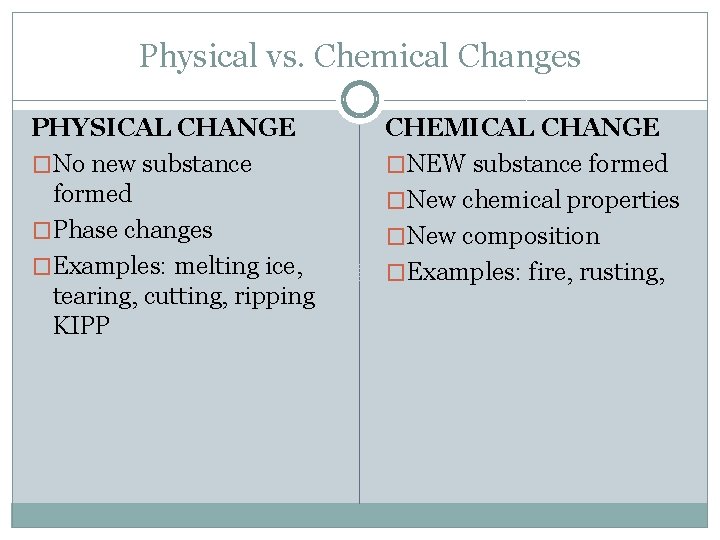
Physical vs. Chemical Changes PHYSICAL CHANGE �No new substance formed �Phase changes �Examples: melting ice, tearing, cutting, ripping KIPP CHEMICAL CHANGE �NEW substance formed �New chemical properties �New composition �Examples: fire, rusting,

Balancing Chemical Equations �Come to tutoring if you don’t know how to do this! �YOU MUST KNOW HOW TO DO THIS! You WILL FAIL THE FINAL IF YOU DON’T KNOW HOW TO BALANCE A CHEMICAL EQUATION!
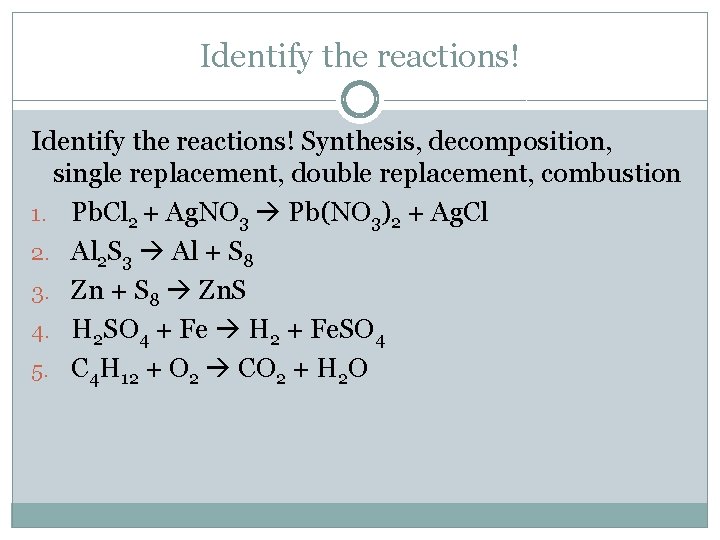
Identify the reactions! Synthesis, decomposition, single replacement, double replacement, combustion 1. Pb. Cl 2 + Ag. NO 3 Pb(NO 3)2 + Ag. Cl 2. Al 2 S 3 Al + S 8 3. Zn + S 8 Zn. S 4. H 2 SO 4 + Fe H 2 + Fe. SO 4 5. C 4 H 12 + O 2 CO 2 + H 2 O
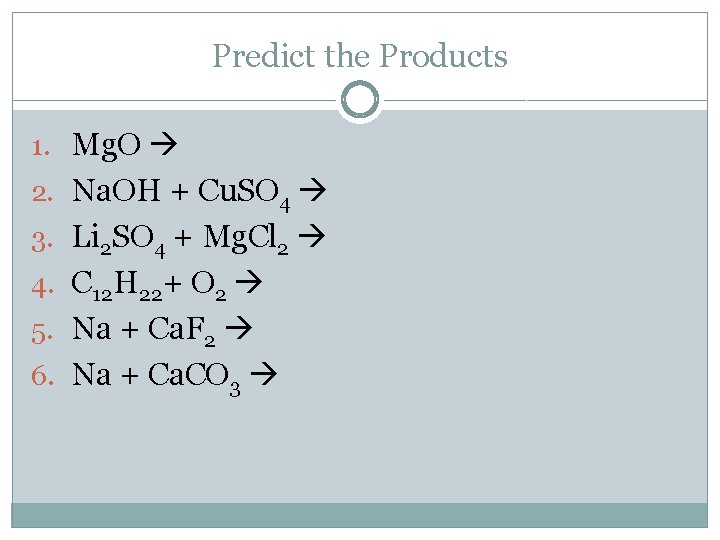
Predict the Products 1. Mg. O 2. Na. OH + Cu. SO 4 3. Li 2 SO 4 + Mg. Cl 2 4. C 12 H 22+ O 2 5. Na + Ca. F 2 6. Na + Ca. CO 3

Stoichiometry Tips �In every problem, you are going to start with your given at the top left! No matter what! �You want all unwanted units to cancel! This means they need to be diagonal to each other. �You cannot go from moles of one substance to grams of another in one conversion factor!
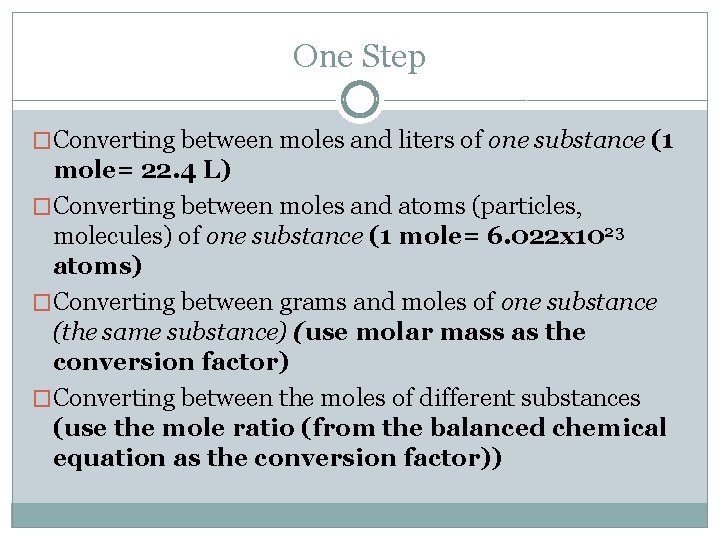
One Step �Converting between moles and liters of one substance (1 mole= 22. 4 L) �Converting between moles and atoms (particles, molecules) of one substance (1 mole= 6. 022 x 1023 atoms) �Converting between grams and moles of one substance (the same substance) (use molar mass as the conversion factor) �Converting between the moles of different substances (use the mole ratio (from the balanced chemical equation as the conversion factor))
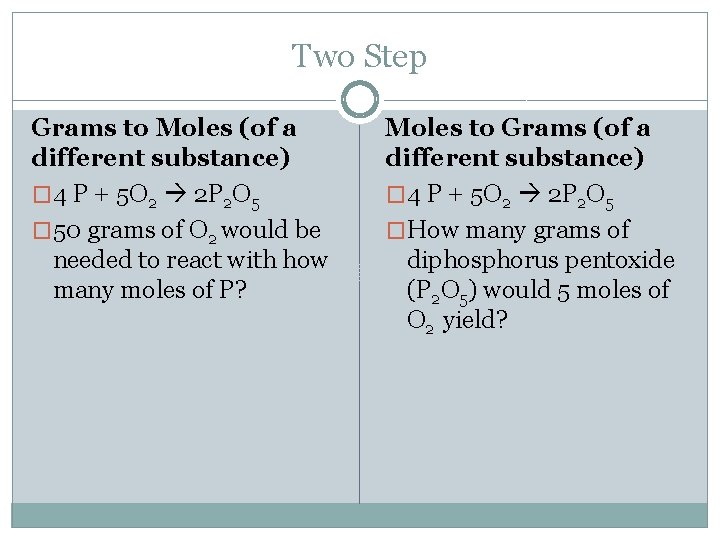
Two Step Grams to Moles (of a different substance) � 4 P + 5 O 2 2 P 2 O 5 � 50 grams of O 2 would be needed to react with how many moles of P? Moles to Grams (of a different substance) � 4 P + 5 O 2 2 P 2 O 5 �How many grams of diphosphorus pentoxide (P 2 O 5) would 5 moles of O 2 yield?
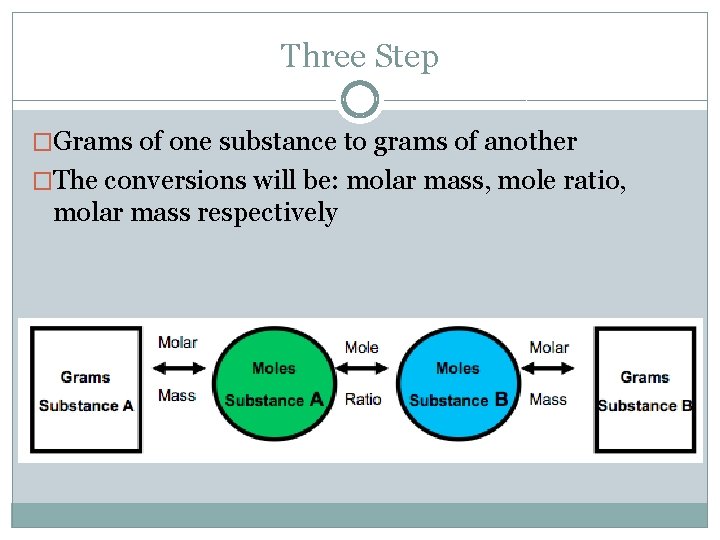
Three Step �Grams of one substance to grams of another �The conversions will be: molar mass, mole ratio, molar mass respectively
- Slides: 28