CASTING TECHNIQUES PDT 361 Technical Ceramic Processing By

CASTING TECHNIQUES PDT 361 - Technical Ceramic Processing By : Pn. Noorina Hidayu Jamil Department of Mechanical Engineering Technology Faculty of Engineering Technology
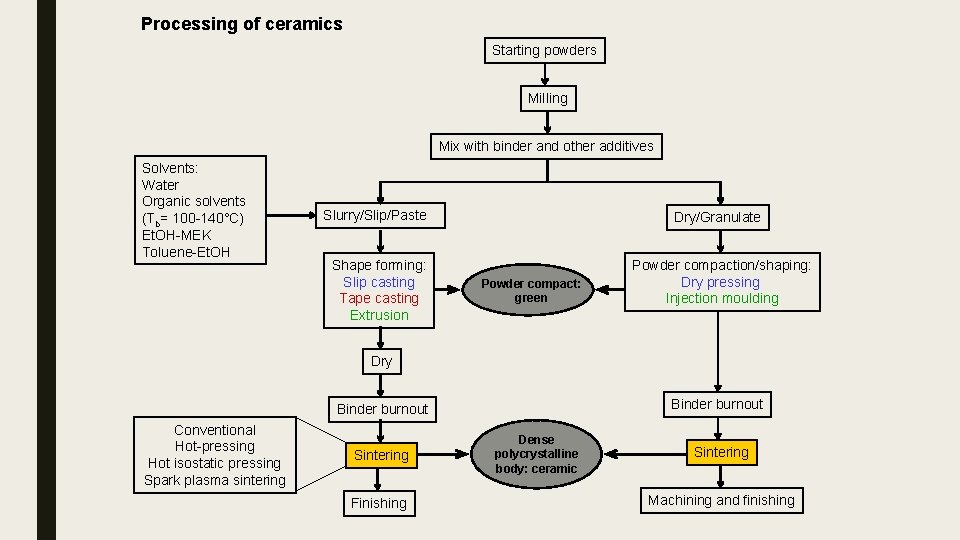
Processing of ceramics Starting powders Milling Mix with binder and other additives Solvents: Water Organic solvents (Tb= 100 -140°C) Et. OH-MEK Toluene-Et. OH Slurry/Slip/Paste Shape forming: Slip casting Tape casting Extrusion Dry/Granulate Powder compact: green Powder compaction/shaping: Dry pressing Injection moulding Dry Binder burnout Conventional Hot-pressing Hot isostatic pressing Spark plasma sintering Sintering Finishing Dense polycrystalline body: ceramic Sintering Machining and finishing

The slip casting process • Slip casting is the process of filling a porous mold, usually a gypsum mold, with a ceramic slurry. • The water is removed from the slurry via capillary action through the small pores in the mold. As the water is removed the slurry the ceramic particles are collected against the surface of the mold. • This collection of particles is the wall of the body that is to be produced. This process is allowed to continue until the correct thickness is achieved, after which the remainder of the slip is drained out of the mold. • The green body is dried further and removed from the mold. After the green body is removed it is dried and fired so that it can go through the final machining process.

Gypsum mold toughness and porosity • The molds used for slip casting usually have a low toughness. • They have a high porosity, which lowers the strength. If the strength is increased some porosity must be sacrificed to increase the strength. • Low strength gypsum molds wear out with time because the pores are eroded from the water that goes through them. • These molds are great for producing complex shapes because as the green body loses water and begins to dry out it shrinks away from the edges of the mold for easy removal. This is good because there is less time lost to parts that are damaged during removal.
![Cast thickness as a function of casting time L=[(2 J∆Pt/n. Rc)+(R`m/Rc) 2]1/2 -(R`m/Rc), • Cast thickness as a function of casting time L=[(2 J∆Pt/n. Rc)+(R`m/Rc) 2]1/2 -(R`m/Rc), •](http://slidetodoc.com/presentation_image_h/32ac363680271834304be5d95a65987a/image-5.jpg)
Cast thickness as a function of casting time L=[(2 J∆Pt/n. Rc)+(R`m/Rc) 2]1/2 -(R`m/Rc), • L = cast thickness • J=vol. of cast/vol. of liq. Removed (inverse of packing factor), • Rc=resistivity to liq. transport in the cast • ∆P=apparent mold suction • n=viscosity of liq. Transported • Rm=liquid transport resistance of the mold

Capillary suction on the slip ∆P = 2γlvcosø/Rc �∆P=suction, �γ =surface tension �ø=angle �Rc=radius of curvature �The flow of liquid through a porous medium is: d. V/dt = K/n * d. P/dx �d. P/dx = the pressure gradient across the filter �n = filtrate viscosity, �d. V/dt = volumetric flow rate of the filtrate and K is the filter permeability

Steps in Slip Casting ■ A plaster of paris mold is prepared. ■ Plaster of paris is porous and absorbs water. ■ A slurry of the powder and water is prepared, which is called the slip. ■ The slip is poured into the plaster of paris mold. ■ The mold absorbs the water and the powder is deposited on the inside of the mold. ■ The mold is split and the powder compact is removed. ■ The powder compact is sintered. ■ Slip casting allows complex shaped parts to be fashioned. Toilet bowls and sinks (sanitary whitewares) are usually made by slip casting.
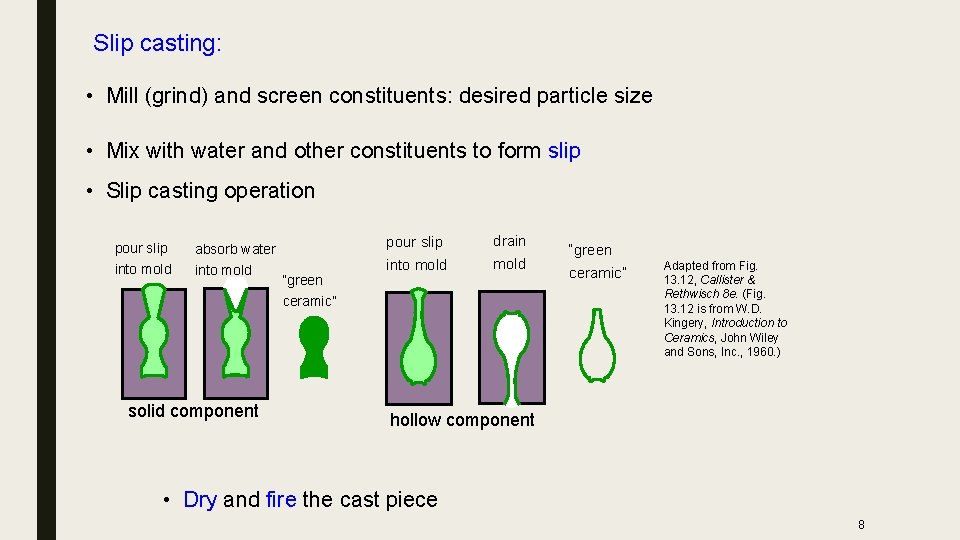
Slip casting: • Mill (grind) and screen constituents: desired particle size • Mix with water and other constituents to form slip • Slip casting operation pour slip into mold absorb water into mold solid component “green ceramic” pour slip drain into mold “green ceramic” Adapted from Fig. 13. 12, Callister & Rethwisch 8 e. (Fig. 13. 12 is from W. D. Kingery, Introduction to Ceramics, John Wiley and Sons, Inc. , 1960. ) hollow component • Dry and fire the cast piece 8
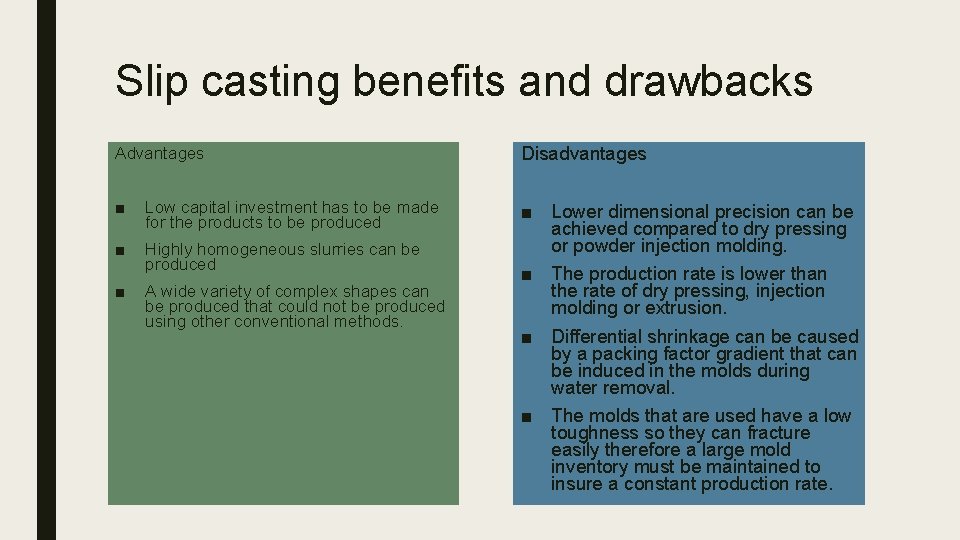
Slip casting benefits and drawbacks Advantages Disadvantages ■ Low capital investment has to be made for the products to be produced ■ Highly homogeneous slurries can be produced ■ A wide variety of complex shapes can be produced that could not be produced using other conventional methods. ■ Lower dimensional precision can be achieved compared to dry pressing or powder injection molding. ■ The production rate is lower than the rate of dry pressing, injection molding or extrusion. ■ Differential shrinkage can be caused by a packing factor gradient that can be induced in the molds during water removal. ■ The molds that are used have a low toughness so they can fracture easily therefore a large mold inventory must be maintained to insure a constant production rate.
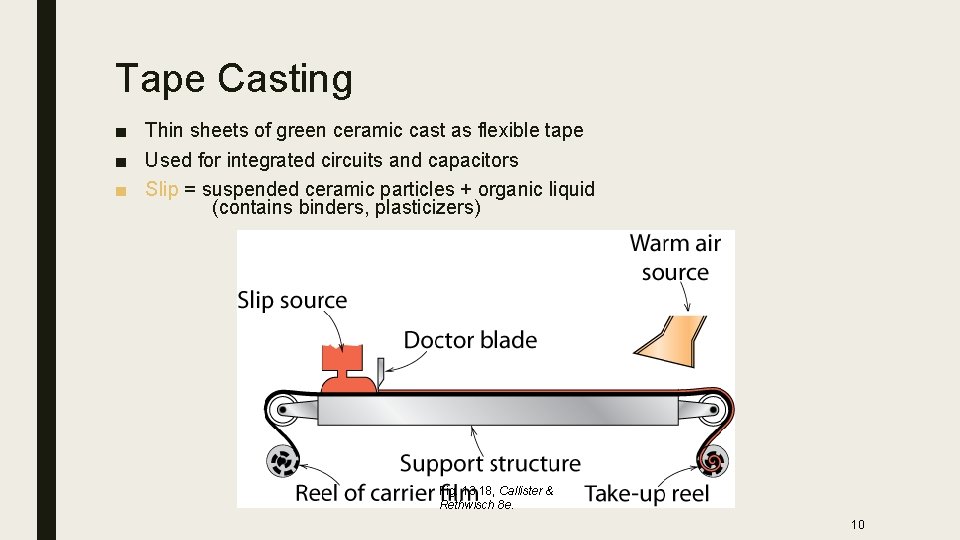
Tape Casting ■ Thin sheets of green ceramic cast as flexible tape ■ Used for integrated circuits and capacitors ■ Slip = suspended ceramic particles + organic liquid (contains binders, plasticizers) Fig. 13. 18, Callister & Rethwisch 8 e. 10
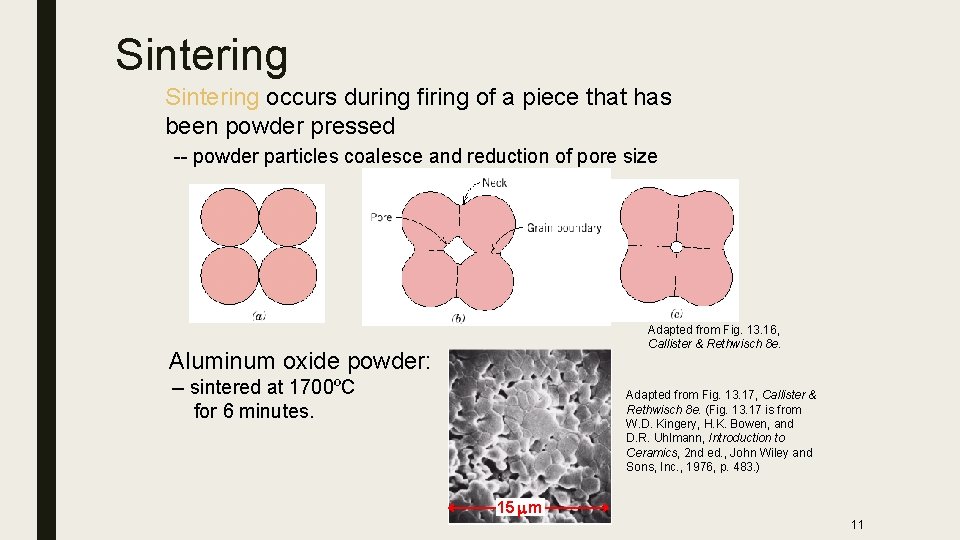
Sintering occurs during firing of a piece that has been powder pressed -- powder particles coalesce and reduction of pore size Adapted from Fig. 13. 16, Callister & Rethwisch 8 e. Aluminum oxide powder: -- sintered at 1700ºC for 6 minutes. Adapted from Fig. 13. 17, Callister & Rethwisch 8 e. (Fig. 13. 17 is from W. D. Kingery, H. K. Bowen, and D. R. Uhlmann, Introduction to Ceramics, 2 nd ed. , John Wiley and Sons, Inc. , 1976, p. 483. ) 15 m 11
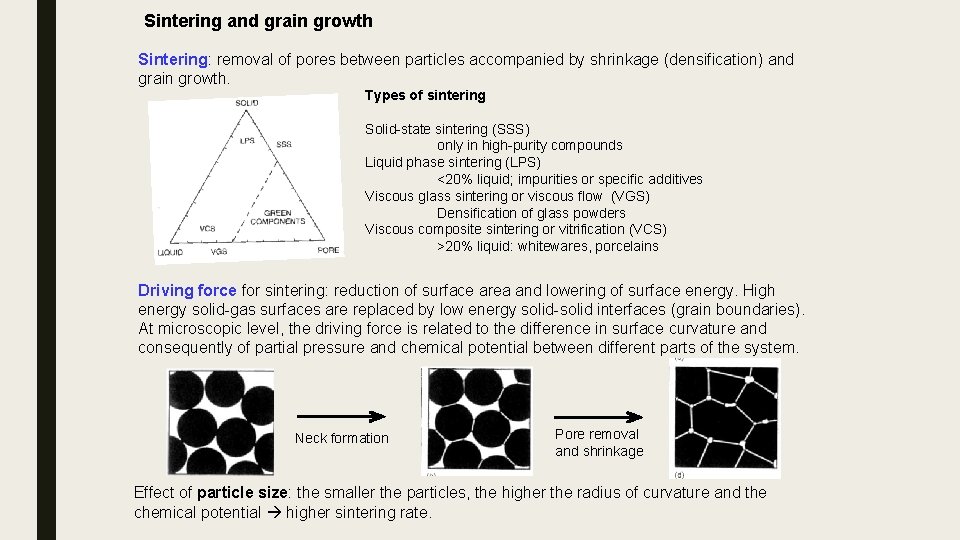
Sintering and grain growth Sintering: removal of pores between particles accompanied by shrinkage (densification) and grain growth. Types of sintering Solid-state sintering (SSS) only in high-purity compounds Liquid phase sintering (LPS) <20% liquid; impurities or specific additives Viscous glass sintering or viscous flow (VGS) Densification of glass powders Viscous composite sintering or vitrification (VCS) >20% liquid: whitewares, porcelains Driving force for sintering: reduction of surface area and lowering of surface energy. High energy solid-gas surfaces are replaced by low energy solid-solid interfaces (grain boundaries). At microscopic level, the driving force is related to the difference in surface curvature and consequently of partial pressure and chemical potential between different parts of the system. Neck formation Pore removal and shrinkage Effect of particle size: the smaller the particles, the higher the radius of curvature and the chemical potential higher sintering rate.
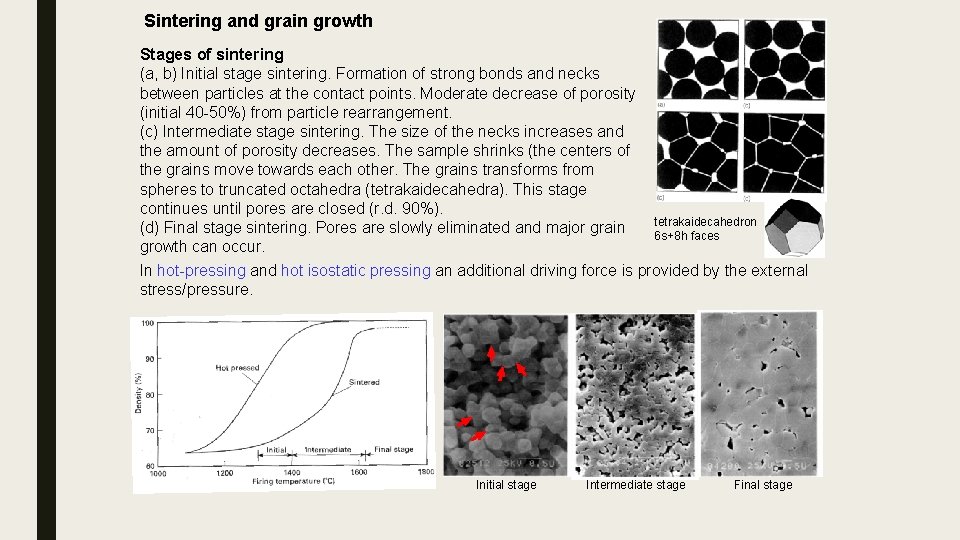
Sintering and grain growth Stages of sintering (a, b) Initial stage sintering. Formation of strong bonds and necks between particles at the contact points. Moderate decrease of porosity (initial 40 -50%) from particle rearrangement. (c) Intermediate stage sintering. The size of the necks increases and the amount of porosity decreases. The sample shrinks (the centers of the grains move towards each other. The grains transforms from spheres to truncated octahedra (tetrakaidecahedra). This stage continues until pores are closed (r. d. 90%). tetrakaidecahedron (d) Final stage sintering. Pores are slowly eliminated and major grain 6 s+8 h faces growth can occur. In hot-pressing and hot isostatic pressing an additional driving force is provided by the external stress/pressure. Initial stage Intermediate stage Final stage
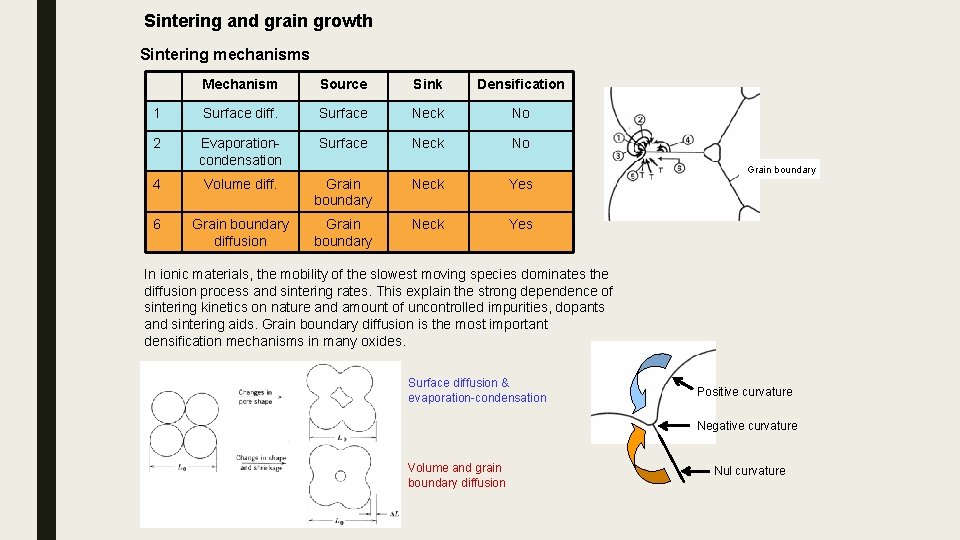
Sintering and grain growth Sintering mechanisms Mechanism Source Sink Densification 1 Surface diff. Surface Neck No 2 Evaporationcondensation Surface Neck No 4 Volume diff. Grain boundary Neck Yes 6 Grain boundary diffusion Grain boundary Neck Yes Grain boundary In ionic materials, the mobility of the slowest moving species dominates the diffusion process and sintering rates. This explain the strong dependence of sintering kinetics on nature and amount of uncontrolled impurities, dopants and sintering aids. Grain boundary diffusion is the most important densification mechanisms in many oxides. Surface diffusion & evaporation-condensation Positive curvature Negative curvature Volume and grain boundary diffusion Nul curvature

- Slides: 15