Case Study Heavy metal bioavailability in a soil

Case Study: Heavy metal bioavailability in a soil affected by mineral sulphides contamination following the mine spillage at Aznalcóllars (Spain) Clemente et al. , Biodegradation, 2003 Aryani Sumoondur Environmental Geosciences, Spring 2005

Los Frailes tailings dam failure, Aznalcóllar, Spain (April, 1998)

Overview n n April 1998: 5 million m 3 of an acidic highly toxic pyrite waste spread along the Guadiamar river and 45 km 2 of arable land solid phase (9 × 105 m 3) spread 37 km downstream Minerals % Pyrite(Fe. S 2) 83. 1 Sphalerite (Zn, Fe)S 5. 4 Galena( Pb. S ) 2. 1 Chalcopyrite(Cu. Fe. S 2) 1. 4 Arsenopyrite(Fe. As. S ) 0. 9 Several trace metals N/A Table 1: Composition of Sludge
Effect on Soil n n n In some areas, heavy metal levels (esp. Zn, Cd, Cu) still present at phytotoxic levels even though most of the sludge and the topsoil was removed Source (Zn, Cd, Cu) : solution phase of spill and solid phase for the other elements Under suitable aeration + moisture conditions, sulphides are oxidised to H 2 SO 4(lower p. H!) 4 Fe. S 2 + 14 H 2 O + 15 O 2 → 4 Fe(OH)3 + 8 SO 42 -+ 16 H+

Aim of Study n n Assess effect of organic amendment and lime (Ca. O) addition on the bioavailability of heavy metals in soils contaminated by the mine spill Factors controlling the solubility and bioavailability of heavy metals 1) Soil p. H 2) Redox potential 3) Soil texture 4) Electrical Conductivity 5) Organic matter (OM) content n 14 months field experiment where the evolution of soil p. H and sulphate formation were monitored in particular

How to study bioavailabilty? n n n Metal fractions are bioavailable when they are in chemical forms which can be taken up by soil organisms and plants Common method: use a chemical extractant or sequential leaching to predict bioavailability of toxic metals in soils Particular chemical phases of metals in the soil are extracted, which correlate well with amounts of metals taken up by plants grown in the soil
Methods and Sampling n n Soil type: non-calcareous, 19. 7% clay, 34. 3% silt, 46% sand ~ 1. 1% OM Treatment : 12 plots of 32 m 2 n n n 3 plots: cow manure (soluble and easily mineralisable OM) 3 plots: mature compost with highly humified OM rest: control lime: applied to highly acidic plots 2 crops of Brassica juncea were grown 2 organic amendments were added 1 month before each sowing and fertilized n n n n After 1 st crop, all plots were divided into 2 -3 subplots due to the great variation of contamination and p. H within plots Plots showing excessive soil acidification were limed p. H to about 6. 0 0– 20 cm deep samples were taken on March, May and Dec 2000 and April 2001 Samples were air dried and sieved at <2 mm

Analytical Methods n n n n Total metal conc. in plant material and soil were determined following HNO 3/HCl. O 4 digestion Bioavailable metals were analysed after extraction with DTPA-Ca. Cl 2 -triethanolamine Analysis: Atomic Absorption Spectrometry (AAS) Soil p. H was measured in a saturated soil paste EC was determined in a 1: 5 aqueous soil extract SO 42 - content was determined by turbidimetry with Ba. Cl 2 Plant growth(fresh and dry weight) were also determined
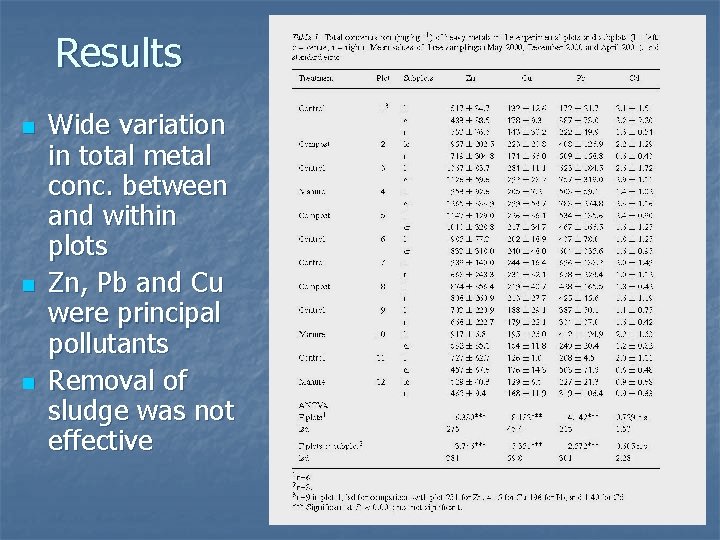
Results n n n Wide variation in total metal conc. between and within plots Zn, Pb and Cu were principal pollutants Removal of sludge was not effective
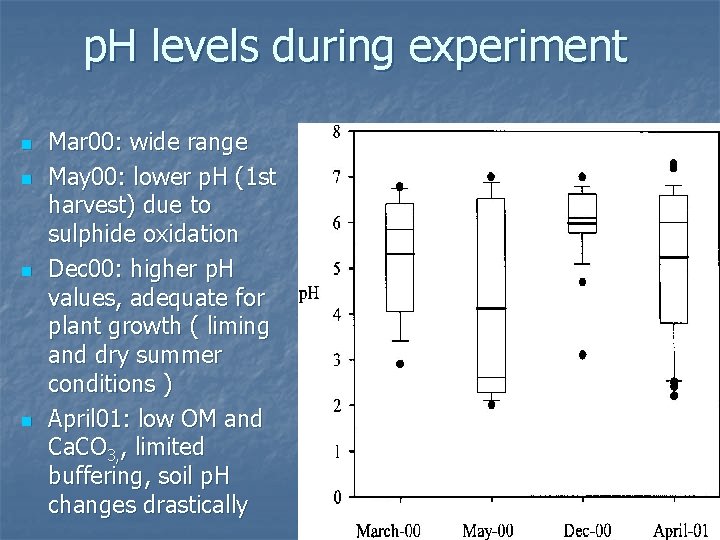
p. H levels during experiment n n Mar 00: wide range May 00: lower p. H (1 st harvest) due to sulphide oxidation Dec 00: higher p. H values, adequate for plant growth ( liming and dry summer conditions ) April 01: low OM and Ca. CO 3, , limited buffering, soil p. H changes drastically
![SO 42 - , EC and p. H n n n [SO 42 -] SO 42 - , EC and p. H n n n [SO 42 -]](http://slidetodoc.com/presentation_image/12c74a47c460f54c54d6cc6a373475dc/image-11.jpg)
SO 42 - , EC and p. H n n n [SO 42 -] affected p. H values of the soil p. H decreased due to sulphide oxidation [SO 42 -] show a close relationship with EC Plots with p. H 7 have lowest [SO 42 -] Liming decreased [SO 42 -] by increasing p. H and precipitation of soluble SO 42 - as Ca. SO 4
In April 2001, sulphate concentrations were at the lowest level With time, the concentration of oxidisable sulphides decreased, which contributed to p. H stabilisation OM which is more readily oxidised could also have affected the redox conditions by reducing sulphide oxidation
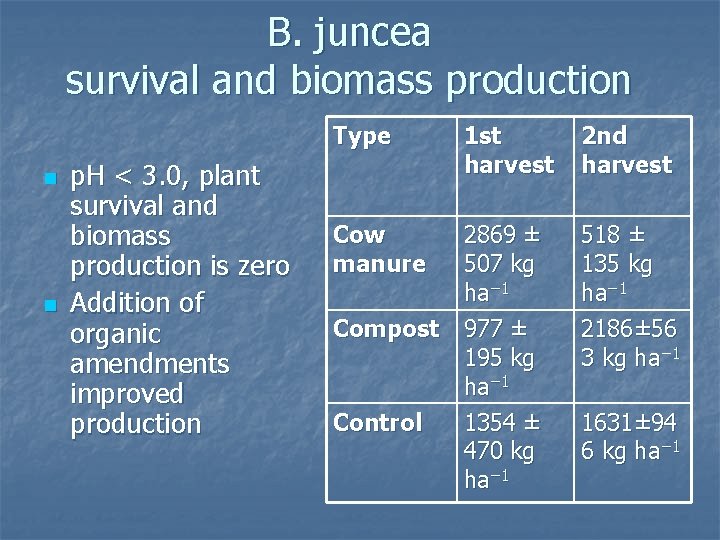
B. juncea survival and biomass production Type n n p. H < 3. 0, plant survival and biomass production is zero Addition of organic amendments improved production Cow manure 1 st harvest 2869 ± 507 kg ha− 1 Compost 977 ± 195 kg ha− 1 Control 1354 ± 470 kg ha− 1 2 nd harvest 518 ± 135 kg ha− 1 2186± 56 3 kg ha− 1 1631± 94 6 kg ha− 1
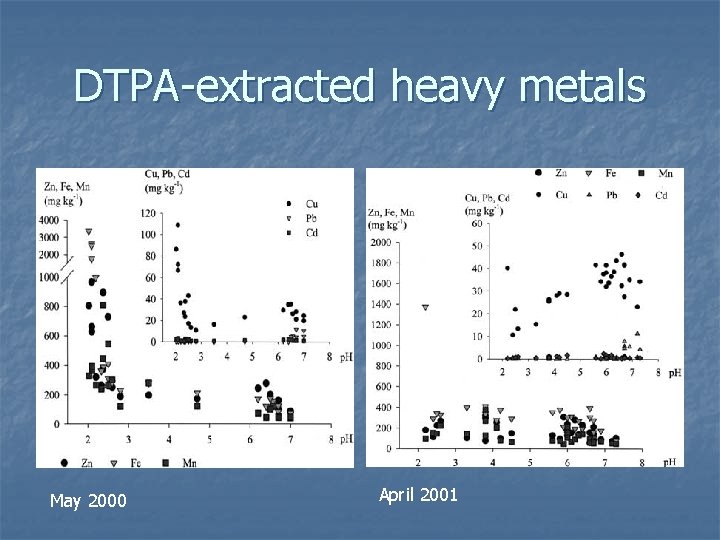
DTPA-extracted heavy metals May 2000 April 2001

Behaviour of different heavy metals n n n Zn, Cu, Fe, Mn are in a wide range in all samplings due to the differing total metal concentrations in each plot After 1 st harvest, highest values of Zn and Cu were found in zones of very low p. H After the 2 nd harvest, soil conc. of Zn, Fe and Mn decreased, even in zones where p. H was low, indicating immobilisation of metals [Zn], [Mn] were directly correlated with [SO 4 2− ] No correlation for [Fe] and [SO 4 2− ], as Fe forms secondary minerals

Behaviour of different heavy metals n n % Pb extracted as low, (0. 8%) although total [Pb] is high Pb shows inverse relationship with [SO 4 2− ] due to formation of insoluble Pb cpds and adsortion on surfaces of Fe-oxides OM generally promoted fixation of heavy metals in non-available soil fractions (Zn decreased from 44. 2% to 26. 7%) Cu bioavailability did not decrease after second harvest due to formation of stable Cu complexes with soluble OM

Conclusions n n Soil was highly contaminated by Zn, Cu and Pb, with a wide range of p. H Plant survival, biomass production and heavy metal contents and bioavailability were conditioned by soil p. H Effect of the organic amendments on the bioavailability of metals was difficult to observe (great variability of total metal concentration and p. H) but OM improved plant growth Liming successfully controlled soil acidification

Effect of OM and lime on soil n n Lime: Raises soil p. H Humified OM and lime immobilise heavy metals, improving soil quality Soluble OM in fresh manure increases short-term solubility of heavy metals However, effect of OM on heavy metal bioavailability in calcareous soils is not related to the OM composition or degree of humification
- Slides: 18