Case Study 2 Covidien Vena Seal Closure System







- Slides: 10

Case Study 2: Covidien Vena. Seal Closure System FDA Perspective Misti Malone, Ph. D. Food and Drug Administration February 24, 2015

Misti Malone, Ph. D. I have no relevant financial relationships

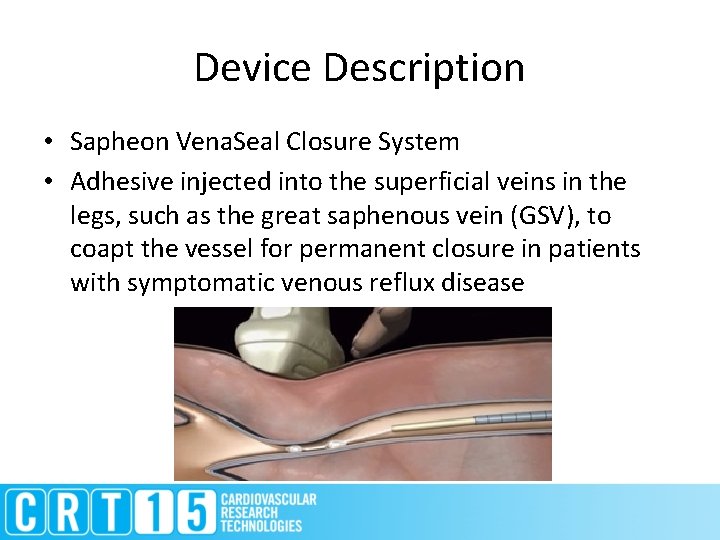
Device Description • Sapheon Vena. Seal Closure System • Adhesive injected into the superficial veins in the legs, such as the great saphenous vein (GSV), to coapt the vessel for permanent closure in patients with symptomatic venous reflux disease

EARLY COMMUNICATION: COME EARLY AND OFTEN

Early Communication • Sept 2011: Sponsor approached DCD at the Tissue Adhesive Symposium • May, July 2012: Pre-IDE Submissions – Regulatory Pathway – Pre-Clinical Testing – Proposed Clinical Study Design

Pre-IDE Interaction Outcome Regulatory Pathway IDE and PMA based on risks similar to currentlyapproved sealants and embolization agents Bench testing Early discussion regarding chemical characterization, biocompatibility, and bench testing to include stability and shelf life testing Clinical Design Randomized Controlled Trial Endpoints Follow-up Blinding 3 -month primary effectiveness endpoint with longterm (12 month) data Consent to 3 years to answer post-approval questions Add a Core Lab to assess outcomes in a blinded fashion

CONTINUED INTERACTION

Pre-PMA Interaction • Modular approach – Focused interaction – Resolved concerns with pre-clinical and manufacturing information prior to final module • Long-term data – Final module contained data up through 6 months – Amendment with 12 -month data by day 60

PMA Interaction • Training Concern – Sponsor provided a demonstration – Various disciplines included as part of clinical trial – Training included as part of distribution • Finalize labeling • Approved Friday, February 20, 2015

Conclusions • Quality, well-written submissions • Maintain momentum • Communicate with the FDA: Early & Often!