Case presentation Eosinophilic gastroenteritis A 35 years old











































- Slides: 54

Case presentation Eosinophilic gastroenteritis

A 35 years old man presented with: abdominal pain in periumbilical non bloody diarrhea(4 -5/day)from one month ago. Weight loss (+)3 -4 kg Fever(-) Nausea and vomiting(-)

PMH: Neg Drug: Metronidazol-Bismuth-Ciprofloxacin. Pantoprazol FH: gastric cancer in his father

V/S: Stable Head and neck: NL Chect: NL Abdomen; mild epigasteric in and preumbilical tenderness

WBC=13300 HB=13. 3 MCV=88 MCH=29 PLT=214000 PMN=70 LYM=25 ESR=8 TSH=4 CRP=17 Amylase=33 Lipase=27 LDH=384 PT=14 INR=1. 1

BS= 126 BUN=18 Cr=1. 7 Ca=8. 6 P=4. 7 Na=138 K=4. 8 AST=15 ALT=10 ALP=133 PRO=5. 2 A lb=3. 2 Total Bil=0. 9 Direct Bil=0. 3

S/E WBC=8 -10 RBC=6 -8

Abdominal&pelvic sonography Normal

Abdominal dupler sonography Normal



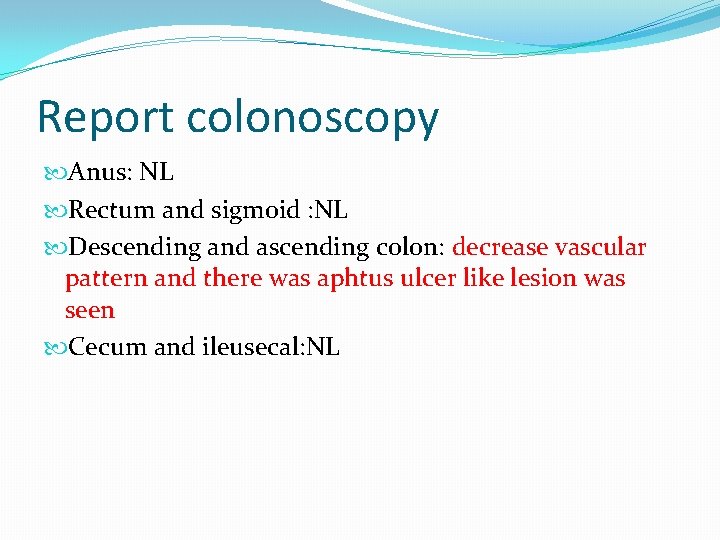
Report colonoscopy Anus: NL Rectum and sigmoid : NL Descending and ascending colon: decrease vascular pattern and there was aphtus ulcer like lesion was seen Cecum and ileusecal: NL

Anti TTG=Neg Anti endomysial=Neg






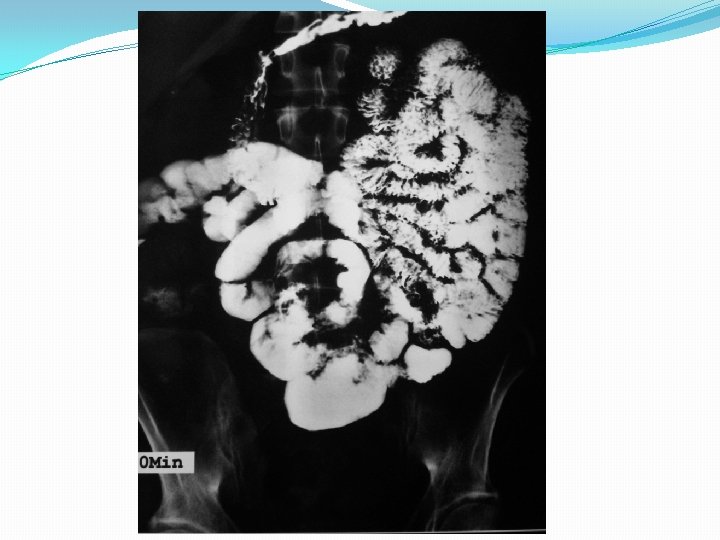
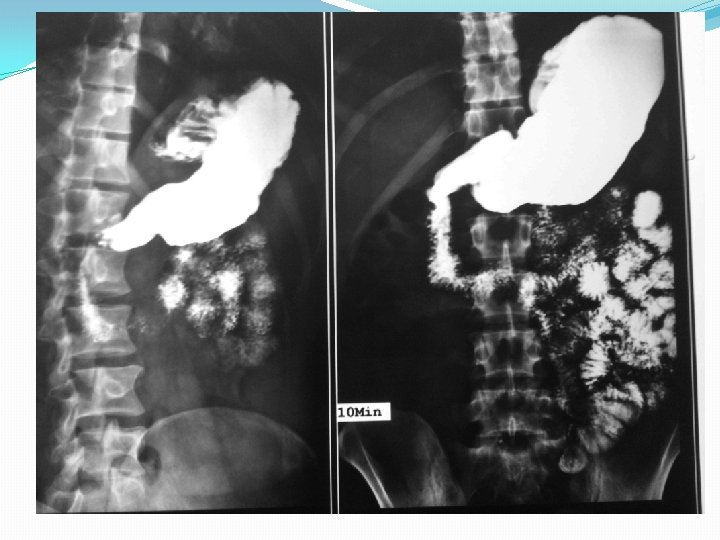
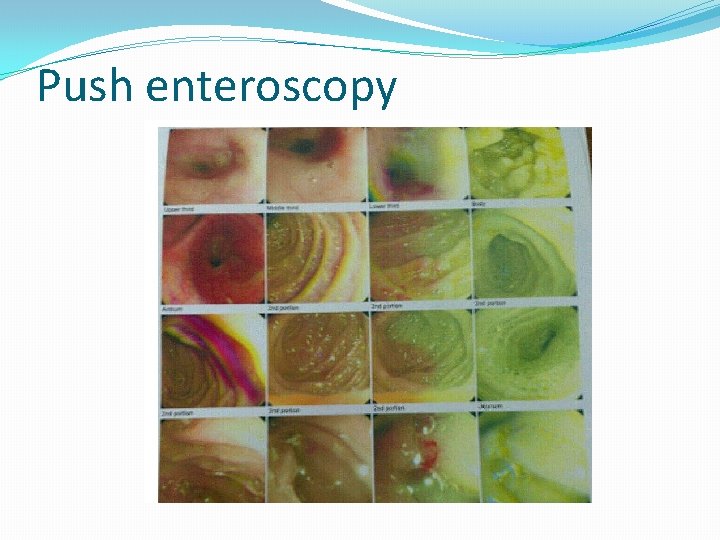
Push enteroscopy



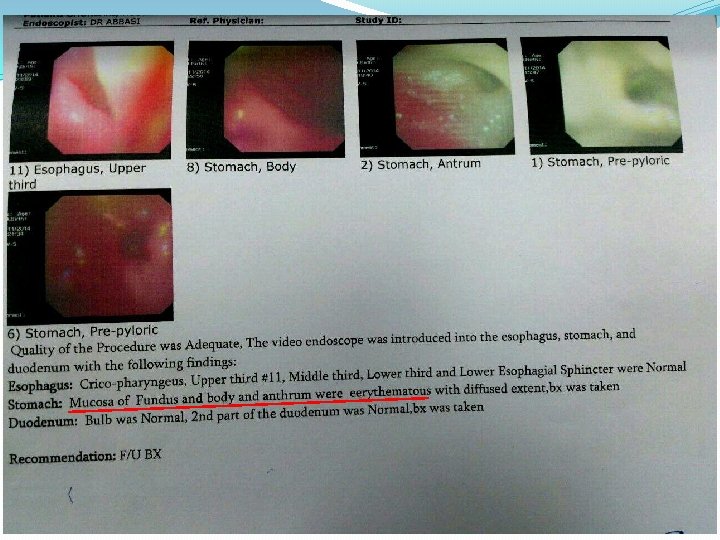
Gastric antral biopsy Antral type mucosa with mild chronic active gastritis , focal intestinal metaplasia, incearesed eosinophilic infiltration

Duodenal BX Small intestinal mucosa with severe eosinophilic infiltration, consistent with eosinophilic entritis

Terminal ileum bx Small intestinal mucosa within normal limits

Eosinophilic gastroenteritis (EG) represents one member of a family of diseases that includes: eosinophilic esophagitis, gastritis, enteritis, and colitis, collectively referred to as eosinophilic gastrointestinal disorders (EGIDs).

Despite its rarity, eosinophilic gastroenteritis needs to be recognized by the clinician because this treatable disease can masquerade as irritable bowel syndrome.

The diagnosis of EG is confirmed by a characteristic biopsy and/or eosinophilic ascitic fluid in the absence of infection by intestinal parasites or other causes of intestinal eosinophilia.

EG has a peak age of onset in the third decade. The clinical features of EG are related to the layer(s) and extent of bowel involved with eosinophilic infiltration: mucosa; muscle; and/or subserosa. The disease can affect patients of any age, but typical presentations are in the third through fifth decade with a male predominance.

Laboratory findings Peripheral eosinophil counts are usually elevated, ranging from 5 to 35 percent with an average absolute eosinophil count of 1000 cells/micro. L, but may be normal in about 20 percent of patients. Patients with malabsorption may have the typical laboratory findings of this disorder: abnormal D-xylose test, increased fecal fat excretion, prolonged PT.

Hypoalbuminemia and anemia can be induced by a protein-losing enteropathy, impaired iron absorption, and occult gastrointestinal bleeding. The ESR is usually normal , but can be elevated modestly in about 25 percent of patients. Serum Ig. E levels can be elevated, especially in children.

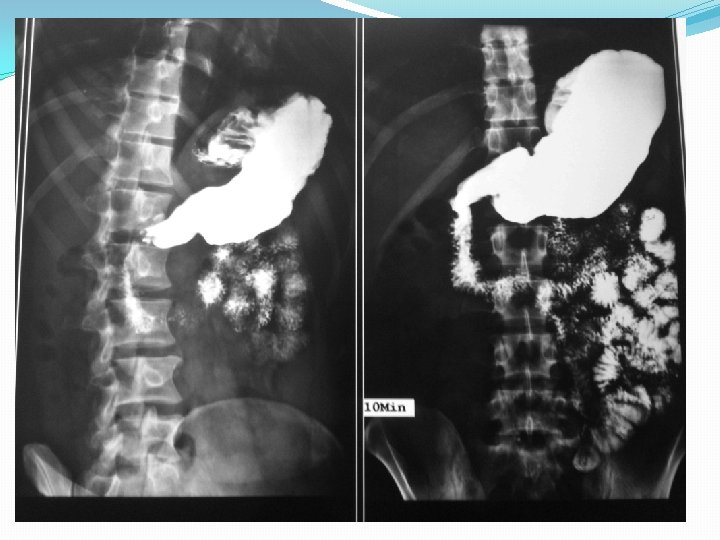
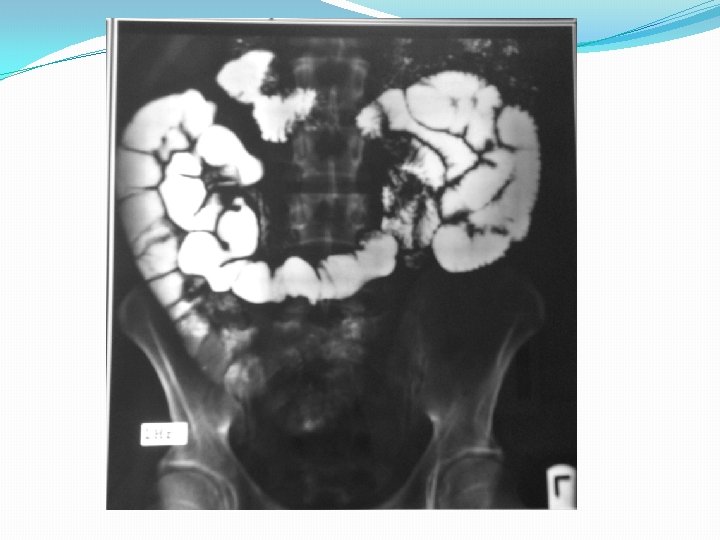
Barium studies of the gastrointestinal tract may suggest the diagnosis but are neither sensitive nor specific. They typically reveal thickening or nodularity in the antrum and a thickened or "sawtooth" mucosa in the small bowel.

Diagnosis The diagnosis of mucosal EG is typically confirmed by endoscopic biopsies, which reveal ≥ 20 to 25 eosinophils per high power field on microscopic examination.

Upper endoscopy with biopsy of the stomach and small intestine is diagnostic in at least 80 percent of patients. Typical endoscopic findings in mucosal disease include nodular or polypoid gastric mucosa, erythema, or erosions.

Biopsies should be taken from both normal and abnormal appearing mucosa because even normal appearing mucosa can demonstrate eosinophilic inflammation. Multiple biopsy samples (at least four to five biopsies per site) should be taken from both the stomach and small intestine including areas with visual abnormalities to overcome sampling error.

PATHOGENESIS The pathogenesis of eosinophilic gastroenteritis (EG) is not well understood. Several epidemiologic and clinical features suggest an allergic component: allergic disease, such as asthma, defined food sensitivities, eczema, or rhinitis. Some patients have elevated serum Ig. E levels

DIAGNOSIS Eosinophilic gastroenteritis (EG) should be suspected in any patient with gastrointestinal symptoms associated with peripheral eosinophilia. It should also be considered before making a diagnosis of irritable bowel syndrome.

DIFFERENTIAL DIAGNOSIS intestinal parasites such as Ancylostoma, Anisakis, Ascaris, Strongyloides, Toxocara, Trichiura, Capillaria, and Trichinella all cause eosinophilia and should be excluded with careful examination of the stool for ova or parasites and/or appropriate serologic testing.

Malignancies, such as lymphoma, gastric cancer, and colon cancer, can present with intestinal obstruction, masses on barium radiography, and eosinophilia. They can be differentiated from EG by endoscopic or full thickness biopsy. Conversely, EG can mimic some of the features of a MALT lymphoma, with bowel wall thickening and marked retroperitoneal lymphadenopathy. .

“ Crohn's disease can usually be differentiated by the typical architectural distortion that is not found in EG. Rarely, Crohn's disease or ulcerative colitis may be associated with peripheral eosinophilia and/or an eosinophil rich tissue infiltrate

Polyarteritis nodosa is associated with systemic manifestations, a markedly elevated ESR, and perivascular eosinophilia.

Hypereosinophilic syndrome (HES) is an idiopathic condition associated with marked peripheral eosinophilia and may rarely present with predominantly gastrointestinal symptoms.

Eosinophilic granuloma (Langerhans cell histiocytosis), which can present as an antral mass, is diagnosed by its typical granulomatous appearance on biopsy specimens.

PROGNOSIS AND TREATMENT Untreated patients with EG may rarely remit spontaneously or progress to severe malabsorption and malnutrition. Patients who are symptomatic or have evidence of malabsorption may be treated with systemic glucocorticoids. Micronutrient deficiencies should be sought and replaced as needed.

The empiric elimination diet is similar to the six-food elimination diets employed in Eo. E, with the patient avoiding soy, wheat, corn, egg, milk, peanut, and seafood.

If dietary measures do not result in decreased symptoms and tissue eosinophilia, we suggest a trial of prednisolone (typically 20 to 40 mg/day). Improvement usually occurs within two weeks regardless of the layer of bowel involved.

Prednisone should then be tapered rapidly over the next two weeks. However, some patients require more prolonged therapy (up to several months) to produce resolution of symptoms. Patients not responding to prednisone can be tried on intravenous glucocorticoids.

Some patients have no recurrences , or only require periodic glucocorticoid bursts, The latter patients may require long-term, low-dose maintenance therapy with prednisone (eg, 5 to 10 mg/day). Other patients experience periodic flares months to years after the initial episode. They can be treated with another short course of oral prednisone, 20 to 40 mg/day, followed by a rapid taper

It should be noted that the formulation of budesonide currently available for gastrointestinal use is in controlled ileal release capsules, which largely bypass the upper gastrointestinal tract.

Oral cromolyn (800 mg/day in four divided doses) has been effective for short- and long-term management in some , but conflicting results.

Ketotifen (Zaditen), an H 1 -antihistamine, has been helpful in individual cases. In adults, it is administered at a starting dose of 1 mg at night and increased to 2 to 4 mg per day for one to four months.

The leukotriene antagonist, montelukast, was effective in some reported cases but not in others

A clinical response to suplatast tosilate (a novel antiallergic drug that suppresses cytokine production including interleukin-4 and interleukin-5 from T helper 2 cells) was described in a single patient.

Omalizumab: Tissue eosinophilia was reduced but results were not statistically significant.

In a preliminary report of four patients, treatment with a humanized anti-interleukin-5 antibody was associated with reduced peripheral and tissue eosinophil counts but had no effect on symptoms. Rebound eosinophilia has been observed after the drug was discontinued.