Case Management of Suspect Avian Influenza A H























































![B) Close contact (approach within 1 meter [approx. 3 feet]) of an ill patient B) Close contact (approach within 1 meter [approx. 3 feet]) of an ill patient](https://slidetodoc.com/presentation_image/433706448e17ef736419b44c0b5e8086/image-59.jpg)
























- Slides: 86

Case Management of Suspect Avian Influenza A (H 5 N 1) Virus Infection in Humans Part 1: Background information on clinical features of human infection with avian influenza A (H 5 N 1) viruses May, 2007 1

Learning Objectives • Recognize clinical features of avian Influenza A (H 5 N 1) virus infection in humans • Understand how information about the patient before onset of illness can help you suspect H 5 N 1 virus infection 2

Part 1 Session Outline • Clinical features • Epidemiological Context – Exposure 3

Illness Scenario • Alex sick for three days – – – Fever Headache Cough Shortness of breath Muscle aches Watery diarrhea • No one else sick • Works at poultry farm and handles poultry Question: Is avian Influenza A (H 5 N 1) the most likely cause of Alex’s symptoms? 4

Clinical Features 5

General Information Human influenza Affected Age Groups Estimated Incubation Period • All ages affected Avian Influenza A (H 5 N 1) • Children of all ages • Highest attack rate in • Healthy young children <5 years adults • Most complications in elderly >65 years • Highest CFR in adolescents. and persons with chronic medical conditions • Mean: 2 days • Mean: 2 – 5 days • Range: 1 – 4 days • ≤ 7 days 6

Signs and Symptoms Avian Influenza A (H 5 N 1) Type of infection Lower respiratory Symptoms Fever, Cough, Headache Shortness of breath, difficulty breathing Diarrhea in some cases Hospitalized Patients Pneumonia Hypoxia requiring oxygen and respiratory failure requiring intubation and mechanical ventilation Acute Respiratory Distress Syndrome (ARDS) 7

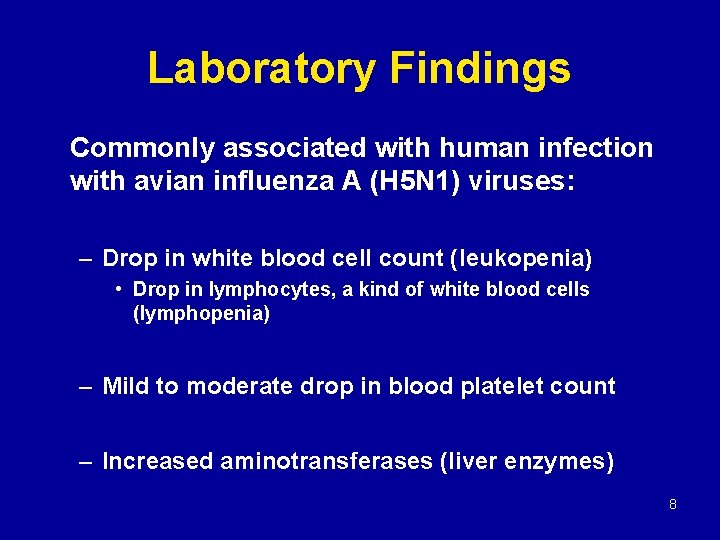
Laboratory Findings Commonly associated with human infection with avian influenza A (H 5 N 1) viruses: – Drop in white blood cell count (leukopenia) • Drop in lymphocytes, a kind of white blood cells (lymphopenia) – Mild to moderate drop in blood platelet count – Increased aminotransferases (liver enzymes) 8

Unusual Clinical Manifestations and Outcomes • Knowledge of avian influenza A (H 5 N 1) virus infection in humans is still evolving • Unusual symptoms – Southern Vietnam – encephalitis and diarrhea – Fever and diarrhea can be the only early signs and symptoms before pneumonia occurs later with H 5 N 1 virus infection 9

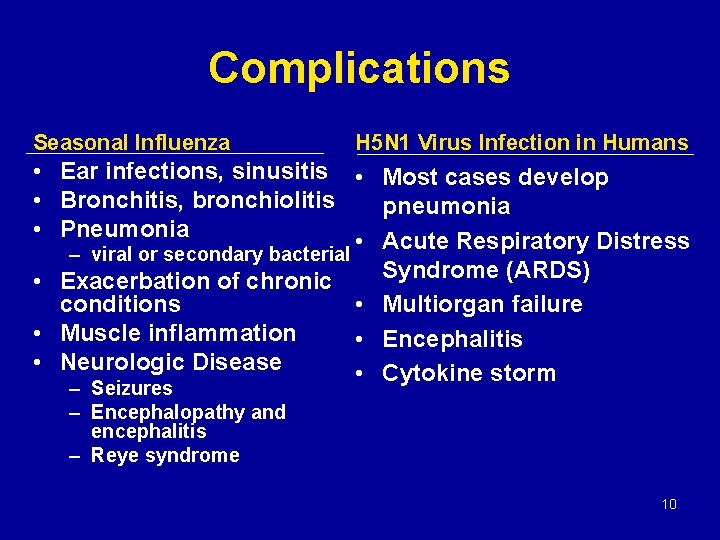
Complications Seasonal Influenza H 5 N 1 Virus Infection in Humans • Ear infections, sinusitis • Most cases develop • Bronchitis, bronchiolitis pneumonia • Pneumonia • Acute Respiratory Distress – viral or secondary bacterial Syndrome (ARDS) • Exacerbation of chronic • Multiorgan failure conditions • Muscle inflammation • Encephalitis • Neurologic Disease • Cytokine storm – Seizures – Encephalopathy and encephalitis – Reye syndrome 10

Alex Question: Do you think Alex has signs and symptoms of H 5 N 1? Why or why not? 11

Epidemiological Context 12

Exposure to Avian Influenza (H 5 N 1) Virus 1. Infected poultry, particularly coming in contact with respiratory secretions 2. Infected wild or pet birds 3. Other infected animals (e. g. , pigs, cats, dogs) 4. Wild bird feces, poultry manure and litter containing high concentrations of virus 5. Fecally contaminated surfaces 13

H 5 N 1 Virus Exposures Continued 6. Under- or uncooked poultry meat or eggs from infected birds 7. Contaminated vehicles, equipment, clothing, and footwear at affected sites, such as poultry farms with outbreaks 8. Contaminated air space (e. g. , a barn, hen-house, or the air space proximal to barn exhaust fans) 9. Bodies of water with infected bird carcasses 10. Close contact with (within 3 feet of) confirmed human cases 14

Local Customs - Unique Exposures • Cock fighting • Swan defeathering • Playing with dead chickens • Duck blood pudding, local customs • Hunting practices 15

Alex Question: Do you think Alex could have been exposed to highly pathogenic avian influenza A (H 5 N 1) virus? 16

Using All of The Information 17

Alex’s Situation • 24 year old Alex sick for three days – – – Fever Headache Cough Shortness of breath Muscle aches Watery diarrhea • No one else sick • Alex works on poultry farm where he handles poultry 18

Alex Question: Would you suspect avian influenza A (H 5 N 1) virus infection? Why or why not? 19

Part 1 Summary • Individuals with avian influenza A (H 5 N 1) virus infection may have nonspecific lower respiratory symptoms, or (rarely) none at all • Ask about recent exposure and contact with humans or animals that may have had avian influenza A (H 5 N 1) virus infection 20

Case Management of Suspect Avian Influenza A (H 5 N 1) Virus Infection in Humans Part 2: Case Management of Suspected Human Cases of Avian Influenza A (H 5 N 1) Virus Infection 21

Learning Objectives • Testing available for diagnosing – Clinical specimens • Current treatment options • Infection control measures 22

Part 2 Session Overview • Laboratory Testing • Treating Suspected Patients • Infection Control in the Healthcare Setting 23

Laboratory Testing 24

Diagnostics • Avian Influenza A (H 5 N 1) Virus – Specimens for testing • Influenza A • Imaging 25

Avian Influenza A (H 5 N 1) Virus Laboratory Tests • RT-PCR – Detects viral RNA – Diagnose H 5 N 1 in humans – BSL-2 conditions – Results within hours • Serologic Testing – Rise in H 5 N 1 specific antibodies – Testing only in enhanced BSL-3 laboratory • Viral culture – Only in enhanced BSL-3 laboratory – Results in 2 -10 days 26

Clinical Specimens for Detecting Avian Influenza A (H 5 N 1) • Lower Respiratory Tract* – – Broncheoalveolar lavage fluid Endotracheal aspirate Pleural fluid Sputum • Upper Respiratory Tract – Oropharyngeal swabs* – Nasal Swab • Collect multiple specimens from the same suspect H 5 N 1 patient on different days for RTPCR testing * Preferred specimens 27

Clinical Specimens for Testing • Serology – Acute and convalescent serum specimens • Acute collected within 1 week of symptom onset • Convalescent collected 2 -4 weeks after symptom onset – Other infections or concurrent illness • Collect all possible specimens, serial collection 28

Clinical Specimens for Testing • Autopsy Specimens – Eight blocks or fixed-tissue specimens from each of the following sites • • Central (hilar) lung with segmental bronchi Right and left primary bronchi Trachea (proximal and distal) Pulmonary parenchyma from both right and left lung – Major organs • Myocardium (right and left ventricle) • CNS (cerebral cortex, basal ganglia, pons, medulla, and cerebellum) • Organ with significant gross or microscopic pathology) 29

Rapid Influenza Diagnostic Tests • NOT RECOMMENDED TO DETECT AND DIAGNOSE H 5 N 1 VIRUS INFECTION – – Many commercial kits available Results in 15 -30 minutes Low sensitivity (FALSE NEGATIVES LIKELY) Positive result cannot differentiate seasonal influenza A from avian influenza A (H 5 N 1) virus infection – Negative result does not rule out avian influenza A (H 5 N 1) virus infection as diagnosis 30

Laboratory Diagnostics • CDC’s influenza laboratory is nation’s influenza reference laboratory • Capable of performing additional tests – Immunohistochemical testing on autopsy specimens • CDC’s Emergency Response Hotline – 770. 488. 7100 31

Imaging Chest X-ray changes of pneumonia are common in the lungs of H 5 N 1 patients • Non-specific changes • Diffuse or patchy infiltrates • Fluid in the space surrounding the lungs • Cavities forming in the lung tissue BBC News. http: //bbb. co. uk Saturday, 3 December 2005 32

Avian Influenza A (H 5 N 1) Virus Patient’s Chest X-Rays Day 5 Day 7 Day 10 Chest x-ray of a patient with avian influenza A (H 5 N 1) virus infection, shown by day of illness Tran Tinh Hien, Nguyen Thanh Liem, Nguyen Thi Dung, et al. New England 33 Journal of Medicine. 18 March, 2004. vol. 350 no. 12. pp 1179 -1188.

Treating Suspected Cases 34

Treatment Options • Antivirals • Supportive care 35

Neuraminidase Inhibitors • Two drugs available – Oseltamivir (Tamiflu®) and Zanamivir (Relenza ®) – Should be given as soon as possible – Effective for treatment and prevention – Used for seasonal influenza and infection with avian influenza A (H 5 N 1) viruses 36

Oseltamivir Dosage for seasonal influenza Adults: 75 mg twice a day for 5 days Children: • <1 year, not recommended • If < 15 kg: the dose should be 30 mg twice a day for 5 days • If >15 kg to <23 kg: the dose should be 45 mg twice a day for 5 days • If >23 kg to <40 kg: the dose should be 60 mg twice a day for 5 days • If >40 kg: the dose should be 75 mg twice a day for 5 days 37

Oseltamivir Treatment for H 5 N 1 patients • Best dosage for H 5 N 1 patients unknown – Consider longer treatment (7 to 10 days) OR – Higher doses (150 mg) – Begin as soon as possible • Dosage for prevention – Once daily for 7 to 10 days after last exposure • Side Effects – Nausea and vomiting – Skin rash – Neurological problems 38

Oseltamivir • Effectiveness in seasonal influenza – Reduces influenza symptoms by 1 day – Reduces some complications of influenza • Cautions- Consider Risk versus Benefits – People with kidney disease (adjust dose) – Pregnant or nursing females • Contraindication – <1 year of age – Hypersensitivity to any component of product • Resistance – Detected in some H 5 N 1 patients 39

Zanamivir • Inhaled by mouth via special device • May be used for treatment of influenza >7 years of age • Treatment dosage – Once in morning and night, 5 days • Side effects – Wheezing, and breathing problems 40

Zanamivir • Effectiveness in seasonal influenza – Reduces influenza symptoms by one day – Reduces lower respiratory tract complications • Consider Risk vs. Benefit – People with chronic respiratory disease – Pregnant or nursing females • Resistance – Very rare in human cases of avian influenza A (H 5 N 1) virus infections – Active against Oseltamivir resistant H 5 N 1 viruses 41

Other Treatments? • Amantadine and Rimantadine – Some H 5 N 1 viruses are resistant • Corticosteroids – Not recommended – Only for worsening sepsis with adrenal insufficiency 42

Treating Children • Different Oseltamivir dosage – Based on child’s weight – Not approved in children <1 year • No aspirin for children <18 years of age – Use Acetaminophen or Ibuprofen • Children potentially infectious for longer periods than adults – If child cannot remain hospitalized, educate family about infection control – Source: WHO Writing Group, Emerging Infectious Diseases, Vol. 12, No. 1, January 2006. 43

Antibiotics • Broad-spectrum – Do not use as a prophylactic – Give empiric therapy for suspected bacterial pneumonia • Secondary bacterial infection therapy – Treat with intravenous antibiotics as recommended 44

Supportive Care Hospital care for suspected or confirmed avian influenza A (H 5 N 1) cases should include: • Isolating the patient • Supplemental oxygen and ventilation • Intensive care support for organ failure 45

Infection Control in Health Care Setting 46

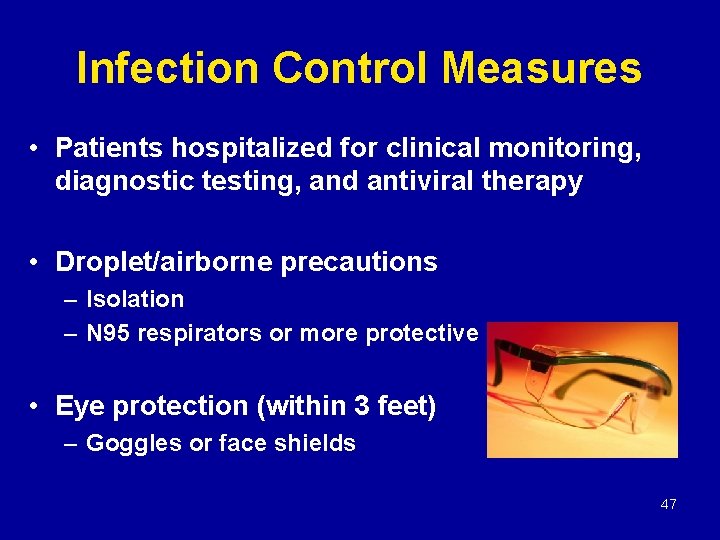
Infection Control Measures • Patients hospitalized for clinical monitoring, diagnostic testing, and antiviral therapy • Droplet/airborne precautions – Isolation – N 95 respirators or more protective • Eye protection (within 3 feet) – Goggles or face shields 47

Infection Control Measures • Standard Precautions – Hand washing before and after contact with patient or potentially contaminated items • Contact Precautions – Gloves and gown worn – Dedicated equipment used • CDC recommendations http: //www. cdc. gov/flu/avian/professional/infect -control. htm 48

Managing Corpses • No known risk of transmission from dead bodies • Autopsy procedures could result in transmission – Use appropriate protective equipment • You should know – Where corpses may be sent for disposal – Cultural or religious beliefs to respect when handling corpses 49

Part 2 Summary 1. Important appropriate clinical specimens are collected and tested 2. Begin treatment with neuraminidase inhibitor (oseltamivir) immediately! Do not wait! 50

Case Management of Suspect Avian Influenza A (H 5 N 1) Virus Infection in Humans Part 3: Public Health Action 51

Learning Objectives • Understand case management from public health perspective • Recognize opportunities for public health authorities to effectively communicate avian influenza A (H 5 N 1) information 52

Part 3 Session Overview 1. Collect Case Information • Classify case according to case definition for surveillance 2. Facilitate specimen collection and laboratory testing 3. Information on avian influenza A (H 5 N 1) illness 4. Infection control measures in the home 5. Active case follow up 6. Identify close contacts and recommend antiviral chemoprophylaxis (oseltamivir) 7. Enhance surveillance 53

Pandemic Influenza Plan • Know your role and responsibilities as outlined in your health department’s plan • Identify key collaborators before and during investigation 54

Collecting Case Information 55

Case Information • Name of person reporting • Healthcare facility name and location • Patient information: Name and contact Information Unique Identifier Occupation (address) Demographic Symptoms Test Results Treatment Outcome Travel history Potential exposures Close contacts 56

Updated Interim Guidance for Laboratory Testing of Persons with Suspected Infection with Avian Influenza A (H 5 N 1) Virus in the United States Testing for avian influenza A (H 5 N 1) virus infection is recommended for a patient who has an illness that: • requires hospitalization or is fatal; AND • has or had a documented temperature of ≥ 100. 4° F; AND • has radiographically confirmed pneumonia, acute respiratory distress syndrome (ARDS), or other severe respiratory illness for which an alternate diagnosis has not been established; AND • has at least one of the following potential exposures within 10 days of symptom onset: 57

A) History of travel to a country with influenza H 5 N 1 documented in poultry, wild birds, and/or humans, AND had at least one of the following potential exposures during travel: • direct contact with (e. g. , touching) sick or dead domestic poultry; • direct contact with surfaces contaminated with poultry feces; • consumption of raw or incompletely cooked poultry or poultry products; • direct contact with sick or dead wild birds suspected or confirmed to have influenza H 5 N 1; • close contact (approach within 1 meter [approx. 3 feet]) of a person who was hospitalized or died due to a severe unexplained respiratory illness; 58
![B Close contact approach within 1 meter approx 3 feet of an ill patient B) Close contact (approach within 1 meter [approx. 3 feet]) of an ill patient](https://slidetodoc.com/presentation_image/433706448e17ef736419b44c0b5e8086/image-59.jpg)
B) Close contact (approach within 1 meter [approx. 3 feet]) of an ill patient who was confirmed or suspected to have H 5 N 1; or C) Worked with live influenza H 5 N 1 virus in a laboratory. 59

Case by Case Considerations! • Mild or atypical disease (hospitalized or ambulatory) with one of the exposures listed above • Severe or fatal respiratory disease whose epidemiological information is uncertain, unavailable, or otherwise suspicious but does not meet the criteria above 60

Proposed Influenza Division/CDC Case Definitions • • Confirmed Suspect Report under investigation Non-case • To be used for reporting purposes • A separate CDC Health Alert Network was released that includes criteria for who should be tested for Influenza A (H 5 N 1) 61

Proposed Influenza Division/CDC Case Definitions Confirmed Case (symptoms, exposure, laboratory confirmation) – Documented temperature >38 C (>100. 4 F) and one of the following: cough, sore throat, and/or respiratory distress AND – One of the following exposures within 10 days of onset • Direct exposure to sick or dead domestic poultry • Direct exposure to surfaces contaminated with poultry feces • Consumption of raw or partially cooked poultry or poultry products • Close contact (within 3 feet) of an ill patient with confirmed or suspected avian influenza A (H 5 N 1) infection • Works with live HPAI (H 5 N 1) virus in a laboratory AND… 62

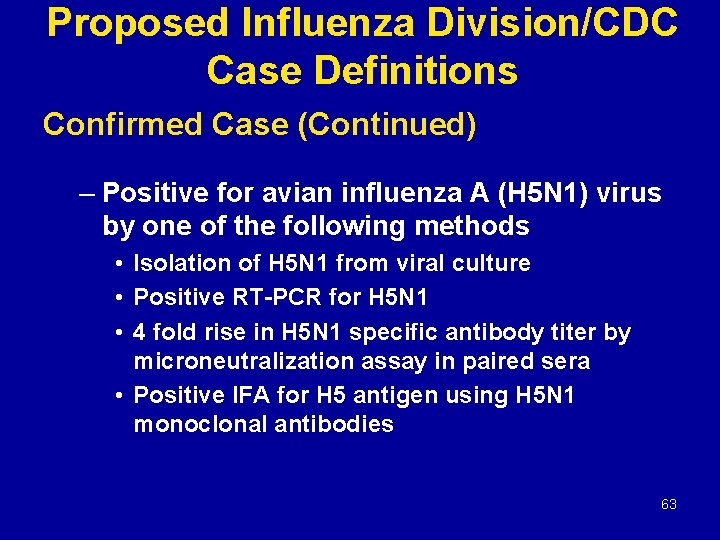
Proposed Influenza Division/CDC Case Definitions Confirmed Case (Continued) – Positive for avian influenza A (H 5 N 1) virus by one of the following methods • Isolation of H 5 N 1 from viral culture • Positive RT-PCR for H 5 N 1 • 4 fold rise in H 5 N 1 specific antibody titer by microneutralization assay in paired sera • Positive IFA for H 5 antigen using H 5 N 1 monoclonal antibodies 63

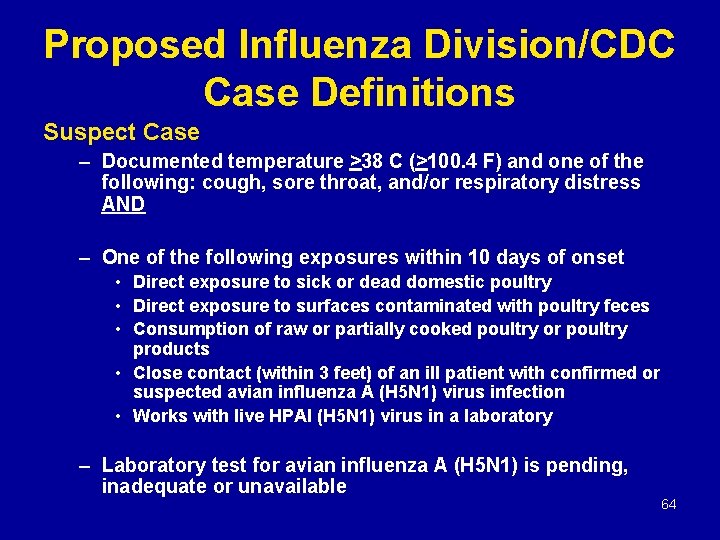
Proposed Influenza Division/CDC Case Definitions Suspect Case – Documented temperature >38 C (>100. 4 F) and one of the following: cough, sore throat, and/or respiratory distress AND – One of the following exposures within 10 days of onset • Direct exposure to sick or dead domestic poultry • Direct exposure to surfaces contaminated with poultry feces • Consumption of raw or partially cooked poultry or poultry products • Close contact (within 3 feet) of an ill patient with confirmed or suspected avian influenza A (H 5 N 1) virus infection • Works with live HPAI (H 5 N 1) virus in a laboratory – Laboratory test for avian influenza A (H 5 N 1) is pending, inadequate or unavailable 64

Proposed Influenza Division/CDC Case Definitions • Report Under Investigation – Additional information needed on clinical and exposure information • Not a Case – Negative avian influenza A (H 5 N 1) virus testing result from a sensitive laboratory testing method using adequate and appropriately timed clinical specimens 65

Reporting • Report through normal channels Local PH State PH CDC • Information shared with WHO—probable and confirmed cases according to WHO case definition • Help determine pandemic phase in US 66

Facilitate Specimen Collection and Laboratory Testing 67

Specimen Collection • Best specimens – Lower respiratory tract • Broncheoalveolar lavage fluid • Endotracheal aspirate • Pleural fluid – Upper respiratory tract • Oropharyngeal swabs • Have supplies stocked for timely collection of appropriate specimens 68

Laboratory Testing • Be familiar with testing available in your area • Know which laboratories can perform which tests • Know tests available at CDC 69

Provide Information on Avian Influenza A (H 5 N 1) Illness 70

Avian Influenza A (H 5 N 1) Virus Infection • Emerging disease with evolving knowledge • Empathy with public concerns • Provide consistent and up to date literature to healthcare providers – Appropriate reading level – Translation for non-English speaking community members • Information such as clinical features, exposure, and treatment options 71

Educate on Infection Control Measures in the Home 72

Infection Control Measures • Give consistent and up to date literature to healthcare providers • Hand washing – Soap and water for 15 -20 seconds – Alcohol based sanitizer, >60% alcohol • Limit close contact with patient • CDC Guidance on Community Mask Use During a Pandemic. www. pandemicflu. gov 73

Infection Control Measures • Seek medical care if condition worsens • Stay home for 24 hours after symptoms resolve • CDC’s recommendation for inhome isolation – http: //www. cdc. gov/ncidod/sars/guid ance/i/pdf/i. pdf 74

Conduct Active Case Follow Up 75

Active Follow Up • Reasons for follow up – – – Specimens for testing Timely notification of results Monitor delivery of antiviral therapy Secure antivirals if shortage Note unusual clinical presentations or complications • Follow up by telephone – Patient – Healthcare provider (when available) – Surrogate (e. g. spouse) 76

Identify Close Contacts 77

Definition of Close Contacts The definition of close contact is household and other contacts in work, school, and community settings who had close unprotected (i. e. , not wearing PPE) contact in the 1 day before through 14 days after the case patient’s symptom onset. Examples of close contact (within 1 meter) with a person include providing care, speaking with, or touching. * http: //www. who. int/csr/resources/publications/influe nza/WHO_CDS_EPR_GIP_2006_4 r 1. pdf * Depending on the specific circumstances suspect or confirmed cases that have completed isolation for at least 7 days, and who are no longer symptomatic, may not be considered a source of exposure to others. 78

Identifying Close Contacts • List of contacts from patient’s case report form • Close contact = within 3 feet or 1 meter – Sharing utensils, close conversation, direct contact • Follow Up – Characterize exposure – Identify signs and symptoms • Those with symptoms treated as potential case of infection with avian Influenza A (H 5 N 1) virus 79

Recommendations to Contacts No symptoms • Post-exposure prophylaxis for close contacts of a strongly suspected or confirmed human case of avian influenza A (H 5 N 1) virus infection – WHO “high” and “moderate” risk categories, poultry depopulators, and responders who have been on infected premises should receive post exposure prophylaxis 80

Instruction to Contacts No symptoms (continued) • Self monitor for 10 days after last exposure – Fever, respiratory symptoms, diarrhea, and/or conjunctivitis – Seek medical care if symptoms present – Notify public health authorities • Follow infection control measures in the home 81

Enhance Surveillance 82

Enhance Surveillance during an Animal or Human Outbreak of Avian Influenza A (H 5 N 1) Virus Infections • Active case finding among occupationally exposed • Sensitization of community to report illness • Expand SARI and/or ILI surveillance to local hospitals, private practice etc. . . – Screening in hospitals • Training on procedures and reporting • Door-to-door community surveillance • Telephone hotlines for reporting 83

Part 3 Summary • Public health authorities serve as protectors of their community’s health • Important that public health authorities provide clear and consistent messages to patients and contacts • Case management also means identifying contacts 84

Glossary Avian Influenza A Viruses Influenza A viruses that cause infection of wild birds and poultry. Contraindication A specific circumstance when the use of a certain treatment could be harmful. Seasonal Influenza Expected rise in influenza occurrence among humans living in temperate climates; occurs during the winter season with strains of human influenza viruses that have minor changes from season to season. 85

References and Resources • CDC Guidance for State and Local Health Departments for Conducting Investigations of Human Illness Associated with Domestic Highly pathogenic Avian Influenza Outbreaks in Animals (Draft). • Preliminary clinical and epidemiological description of influenza A (H 5 N 1) in Viet Nam. 12 February 2004. http: //www. who. int/csr/disease/avian_influenza/guidelines/vietnamclinical/en/in dex. html • Tran Tinh Hien, et al. Avian Influenza A (H 5 N 1) in 10 Patients in Vietnam. N Engl J Med March 18, 2004: 350(12), p 1179 -1181. • WHO interim guidelines on clinical management of humans infected by influenza A(H 5 N 1), 2 March 2004. http: //www. who. int/csr/disease/avian_influenza/guidelines/clinicalmanage/en/in dex. html • WHO pandemic influenza draft protocol for rapid response and containment. Updated draft 30 May 2006. http: //www. who. int/csr/disease/avian_influenza/guidelines/protocolfinal 30_05_0 6 a. pdf 86
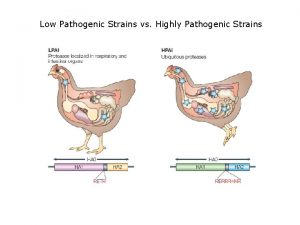
 Low pathogenic avian influenza
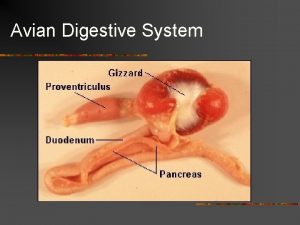
Low pathogenic avian influenza Swine digestive system
Swine digestive system Avian taxonomy
Avian taxonomy Porter novelli healthstyles survey
Porter novelli healthstyles survey Members of an avian species of identical plumage congregate
Members of an avian species of identical plumage congregate Ahas bash
Ahas bash Bam ahas
Bam ahas Bird digestive system functions
Bird digestive system functions Definition of avian
Definition of avian Aves kingdom
Aves kingdom Enantiornithes
Enantiornithes Best worst and average case
Best worst and average case Modulo termico materozza
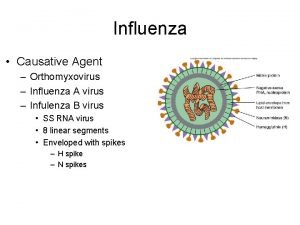
Modulo termico materozza Influenza causative agent
Influenza causative agent The great influenza rhetorical analysis essay
The great influenza rhetorical analysis essay Olfactory mucosa
Olfactory mucosa Influenza
Influenza Stomach flu vs influenza
Stomach flu vs influenza Is influenza a airborne disease
Is influenza a airborne disease Rimantidina
Rimantidina Influenza vaccine dosage chart 2019-2020
Influenza vaccine dosage chart 2019-2020 Influenza ww1
Influenza ww1 Influenza virus replication
Influenza virus replication Black apartheid poems
Black apartheid poems Atypische lymfocyten suspect reactief
Atypische lymfocyten suspect reactief Research question
Research question Which is not a class characteristic of a suspect's sneaker?
Which is not a class characteristic of a suspect's sneaker? Suspect identification
Suspect identification Difference between short case and long case
Difference between short case and long case Average complexity of binary search
Average complexity of binary search Glennan building cwru
Glennan building cwru Bubble sort algorithm pseudocode
Bubble sort algorithm pseudocode Fbi virtual case file case study
Fbi virtual case file case study Bubble sort best case and worst case
Bubble sort best case and worst case Bubble sort best case and worst case
Bubble sort best case and worst case How are the asa case and the saa case differ
How are the asa case and the saa case differ Top management and middle management
Top management and middle management Management pyramid
Management pyramid Top management middle management first line management
Top management middle management first line management Iris ayala
Iris ayala Swanage bay facts
Swanage bay facts Strength based case management
Strength based case management Hospital management er diagram
Hospital management er diagram Use case diagram
Use case diagram Performance management
Performance management Case manager notes examples
Case manager notes examples Cpm matrix of mcdonald's
Cpm matrix of mcdonald's Juvenile case management system
Juvenile case management system Happisburgh erosion case study
Happisburgh erosion case study Gym management system project
Gym management system project Use case diagram for employee management system
Use case diagram for employee management system Planogram software comparison
Planogram software comparison Risk management case study oil and gas industry
Risk management case study oil and gas industry Management information system case study
Management information system case study Management information system case study
Management information system case study Functions of an information system
Functions of an information system Case management monitoring
Case management monitoring Motivational interviewing case management
Motivational interviewing case management Advanced case management
Advanced case management Adaptive case management definition
Adaptive case management definition Acm case management certification study guide
Acm case management certification study guide +reentry +case +management
+reentry +case +management Case study on memory management
Case study on memory management Child protection case management tools
Child protection case management tools Ecm case management
Ecm case management Column case management
Column case management Commcare case management
Commcare case management Assertive case management
Assertive case management Walton on the naze coastal management case study
Walton on the naze coastal management case study Case studies financial management
Case studies financial management Bridge case management
Bridge case management Content management interoperability services
Content management interoperability services Ct violation of probation warrants
Ct violation of probation warrants Management information system case study banking
Management information system case study banking Project scope management case study
Project scope management case study Försäkringkassa
Försäkringkassa Query management and case administration officer
Query management and case administration officer Management functions case
Management functions case Mental health case management
Mental health case management Enterprise risk management at hydro one case study solution
Enterprise risk management at hydro one case study solution Zara strategic management
Zara strategic management Business case for ppm tool
Business case for ppm tool Canadian standards of practice for case management
Canadian standards of practice for case management Case management cos è
Case management cos è Umr case management
Umr case management Proactive case management
Proactive case management Case management involves
Case management involves