CART Cell Therapy Suzanne Schauwecker MD Ph D













- Slides: 26

CAR-T Cell Therapy Suzanne Schauwecker, MD, Ph. D Blood bank rotation presentation 9/20/18

Outline • Mechanism of CAR T cell therapy • Manufacturing process • Challenges of expanding CAR T cell therapy to a larger patient population world-wide • Harmonization of standards nationally and internationally

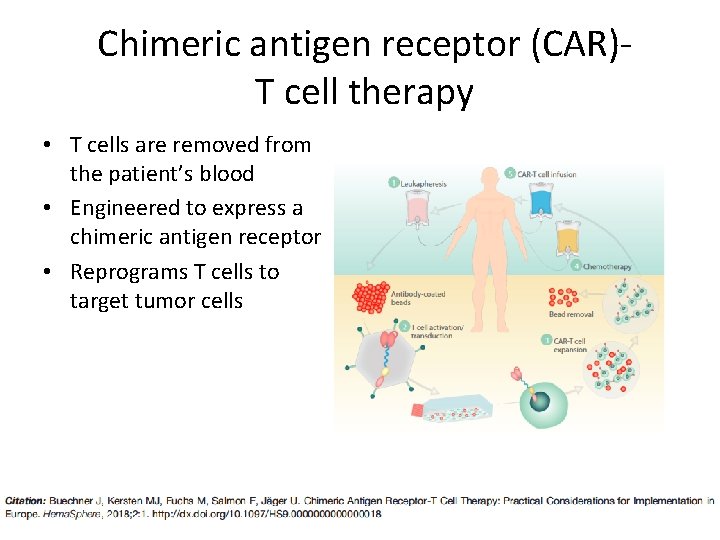
Chimeric antigen receptor (CAR)T cell therapy • T cells are removed from the patient’s blood • Engineered to express a chimeric antigen receptor • Reprograms T cells to target tumor cells

CAR T cell therapy – Where are we? • Tisagenlecleucel (CTL 019) is FDA-approved for patients up to 25 years of age with B-cell precursor ALL (refractory or in second or greater relapse) and for patients with DLBCL (relapsed and refractory) • KTE-C 19/axicabtagene ciloleucel (axi-cel) is FDA-approved for patients with NHL or DLBCL (relapsed and refractory) • Currently being investigated in several hematologic and solid tumor types • 144 current clinical trials in the US, 240 in China, Australia, Austria, Belgium, Canada, France, Germany, Israel, Italy, Japan, Netherlands, Norway, Russia, Singapore, Spain, Sweden, Switzerland, Thailand, United Kingdom

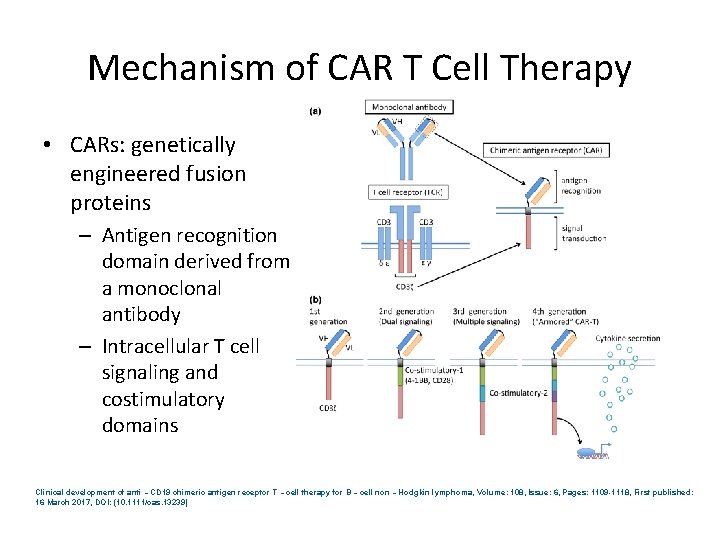
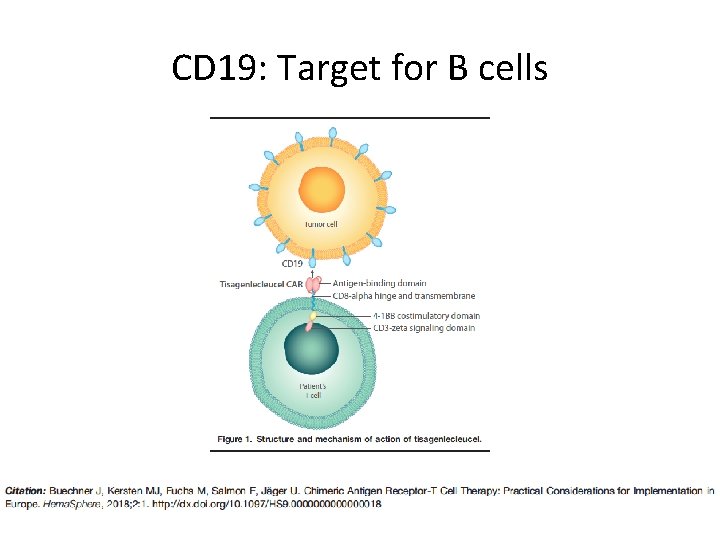
Mechanism of CAR T Cell Therapy • CARs: genetically engineered fusion proteins – Antigen recognition domain derived from a monoclonal antibody – Intracellular T cell signaling and costimulatory domains Clinical development of anti‐CD 19 chimeric antigen receptor T‐cell therapy for B‐cell non‐Hodgkin lymphoma, Volume: 108, Issue: 6, Pages: 1109 -1118, First published: 16 March 2017, DOI: (10. 1111/cas. 13239)

Mechanism of CAR T Cell Therapy • CARs: genetically engineered fusion proteins – Antigen recognition domain derived from a monoclonal antibody – Intracellular T cell signaling and costimulatory domains Clinical development of anti‐CD 19 chimeric antigen receptor T‐cell therapy for B‐cell non‐Hodgkin lymphoma, Volume: 108, Issue: 6, Pages: 1109 -1118, First published: 16 March 2017, DOI: (10. 1111/cas. 13239)

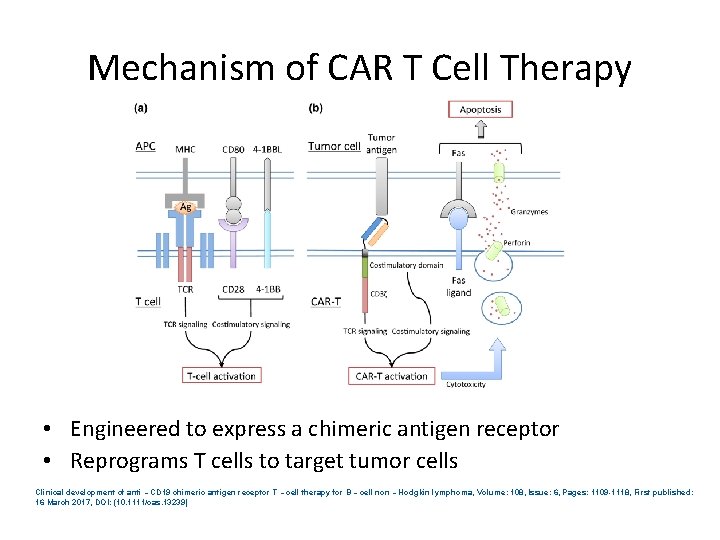
Mechanism of CAR T Cell Therapy • Engineered to express a chimeric antigen receptor • Reprograms T cells to target tumor cells Clinical development of anti‐CD 19 chimeric antigen receptor T‐cell therapy for B‐cell non‐Hodgkin lymphoma, Volume: 108, Issue: 6, Pages: 1109 -1118, First published: 16 March 2017, DOI: (10. 1111/cas. 13239)

CD 19: Target for B cells

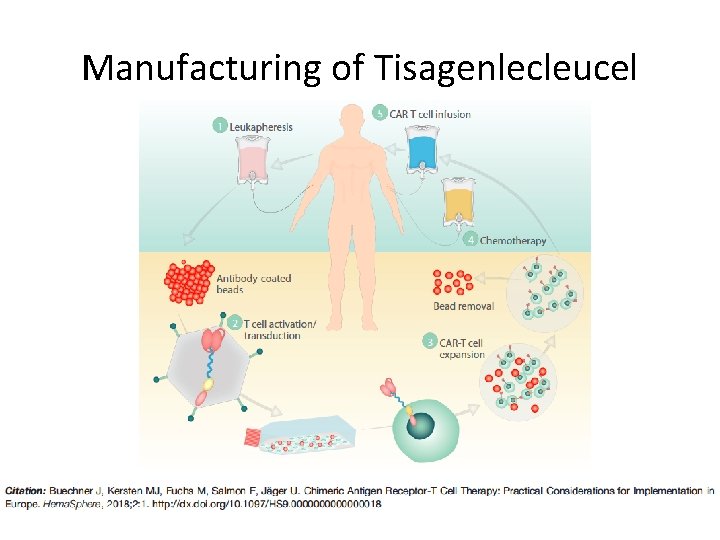
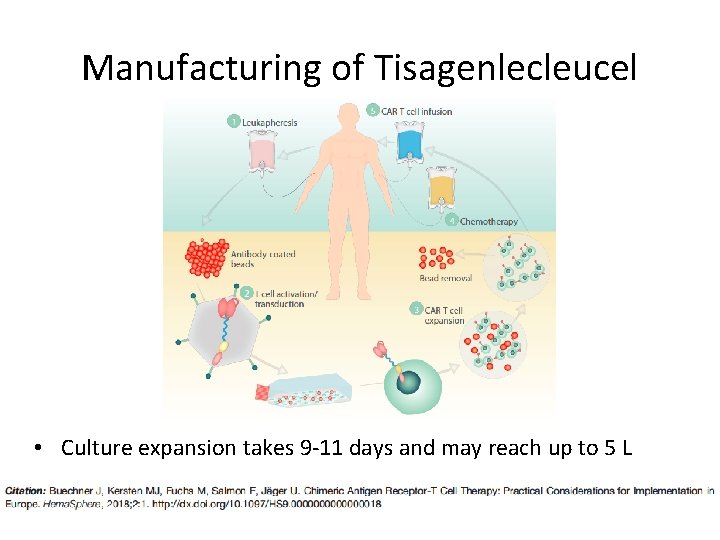
Manufacturing of Tisagenlecleucel

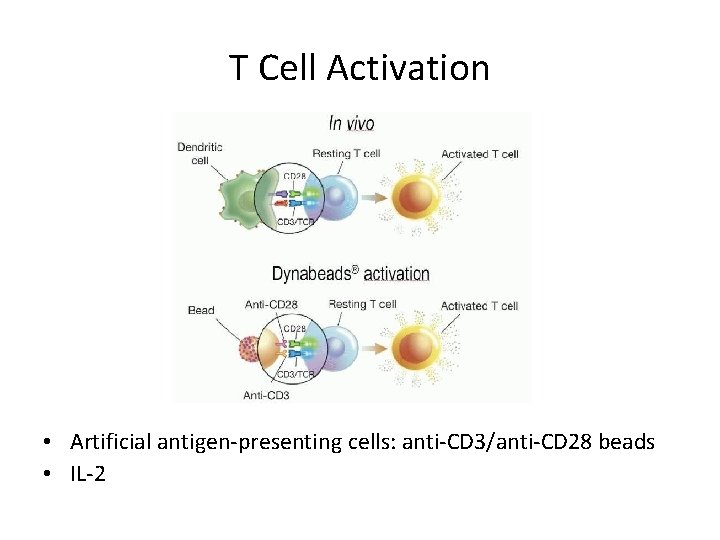
T Cell Activation • Artificial antigen-presenting cells: anti-CD 3/anti-CD 28 beads • IL-2

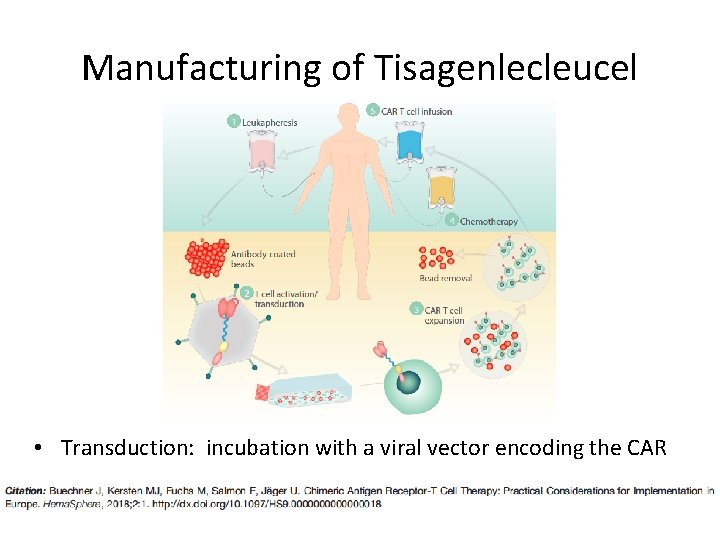
Manufacturing of Tisagenlecleucel • Transduction: incubation with a viral vector encoding the CAR

Manufacturing of Tisagenlecleucel • Culture expansion takes 9 -11 days and may reach up to 5 L

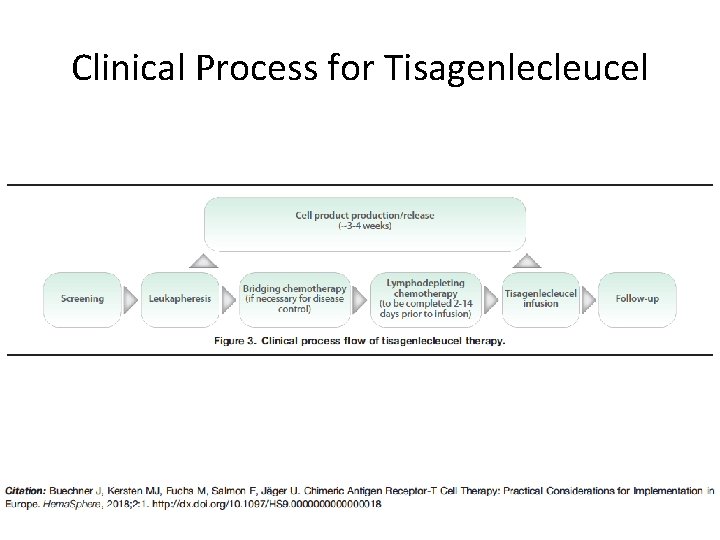
Clinical Process for Tisagenlecleucel

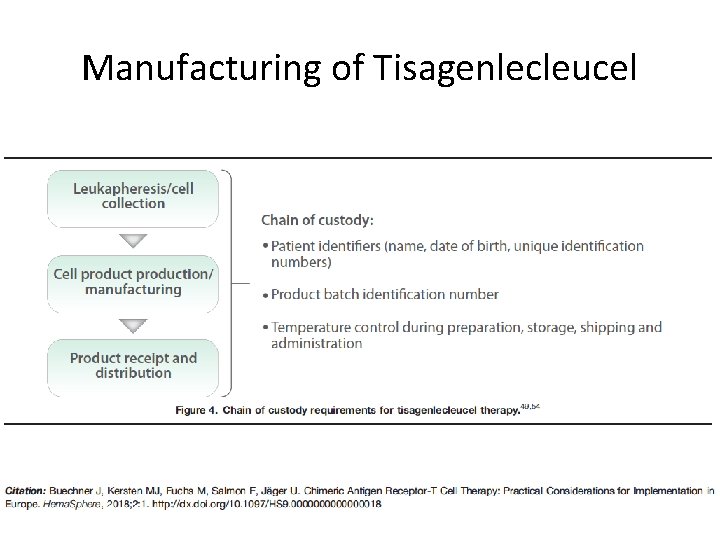
Manufacturing of Tisagenlecleucel

Challenges of Expanding to Larger Patient Populations • Scaling CAR T cell therapy – Wider range of indications – Larger patient populations • Complex manufacturing process – Some aspects are semi-automated – Others are manual • Generating consistently high-quality viral vector – Predictable genetic modification of cells – Sterility • The final CAR T cell product must be individually generated for each patient – Chain of custody regulations • Global regulatory concerns

Challenges of Expanding CAR T Cell Therapy Globally • Donor screening and testing, traceability and labeling, patient confidentiality, and apheresis requirements vary widely among countries • Donor starting material and final product may be shipped internationally • Requirements for quality control of starting materials vary • Requirements for the human or animal serum used for cell culture differ • Greater global regulatory collaboration and harmonization is necessary

Harmonization of Standards • The clinical trial approval process is not yet fully harmonized between European Union member states • This may change in 2019 when a new regulation goes into effect

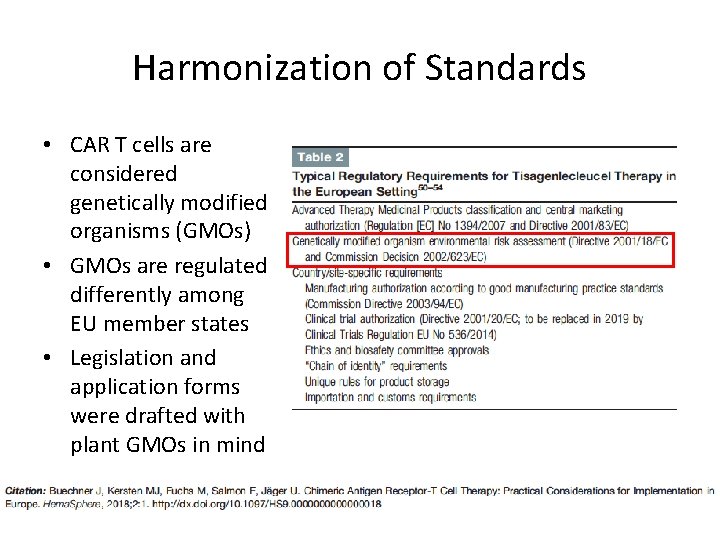
Harmonization of Standards • CAR T cells are considered genetically modified organisms (GMOs) • GMOs are regulated differently among EU member states • Legislation and application forms were drafted with plant GMOs in mind

Harmonization of Standards • GMO applications are often managed by a different government agency than the competent health authority • Some countries require a public consultation – Risk of releasing confidential data to the public

Harmonization of Standards • Different EU member states regard WBCs as blood-derived products vs. tissues and cells for further manufacturing • Various labeling and cell donor testing requirements vary across countries

Harmonization of Standards • NCCN task force report on CAR T cell therapy • Multidisciplinary panel of experts in oncology, cancer center administration, and health policy • Met for the first time in March 2018 • Report published this month

CD 19 CAR T Cell Therapy, Management of Toxicities

Follow-Up

Operational Considerations

Outline • Mechanism of CAR T cell therapy • Manufacturing process • Challenges of expanding CAR T cell therapy to a larger patient population world-wide • Harmonization of standards nationally and internationally

References • • • Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Molecular Therapy: Methods & Clinical Development. 2017; 4: 92 -101. Makita S, Yoshimura K, Tobinai K. Clinical development of anti-CD 19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Science. 2017; 108(6): 1109 -1118. Buechner J, Kersten MJ, Fuchs M, Salmon F, Jager U. Chimeric antigen receptor-T cell therapy: Practical considerations for implementation in Europe. Hema. Sphere. 2018; 2: 1. Ogba N, Arwood NM, Bartlett NL, Bloom M, Brown P, Brown C, Lihua Budde E, Carlson R, Farnia S, Fry TJ, Garber M, Gardner RA, Gurschick L, Kropf P, Reitan JJ, Sauter C, Shah B, Shpall EJ, Rosen ST. Chimeric antigen receptor T cell therapy. NCCN Task Force Report. J Natl Compr Canc Netw 2018; 16(9): 1092– 1106. Locke FL, Davila ML. Regulatory challenges and considerations for the clinical application of CAR-T cell anti-cancer therapy. Expert Opinion on Biological Therapy. 2017; 17(6): 659 -661. Maus MV, Nikiforow S. The why, what, and how of the new FACT standards for immune effector cells. Journal for Immuno. Therapy of Cancer. 2017; 5: 36.