Carnots Theorem We introduced already the Carnot cycle
- Slides: 12

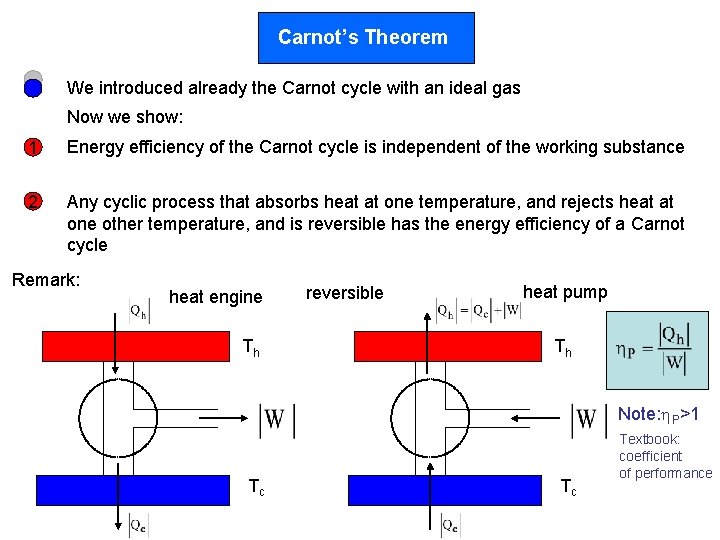
Carnot’s Theorem We introduced already the Carnot cycle with an ideal gas Now we show: 1 Energy efficiency of the Carnot cycle is independent of the working substance 2 Any cyclic process that absorbs heat at one temperature, and rejects heat at one other temperature, and is reversible has the energy efficiency of a Carnot cycle Remark: heat engine Th reversible heat pump Th Note: P>1 Tc Tc Textbook: coefficient of performance

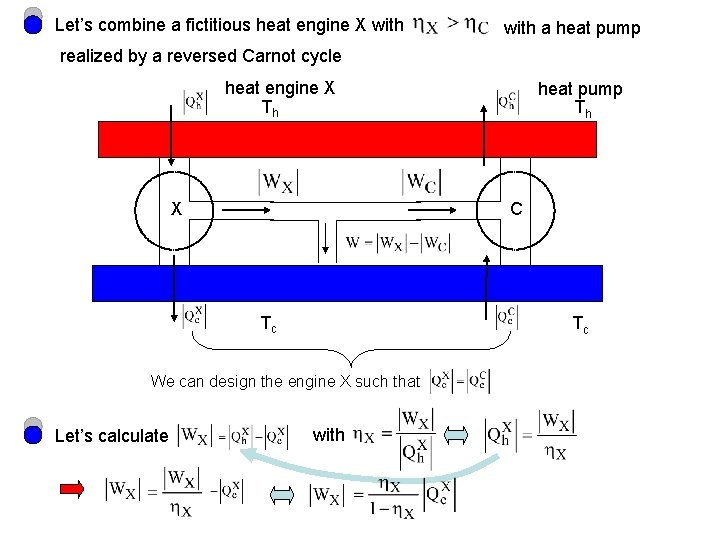
Let’s combine a fictitious heat engine X with a heat pump realized by a reversed Carnot cycle heat engine X Th X C Tc Tc We can design the engine X such that Let’s calculate heat pump Th with

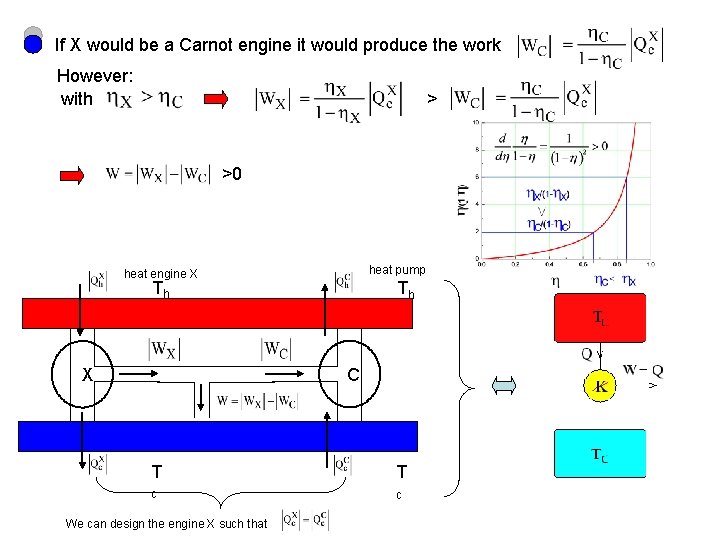
If X would be a Carnot engine it would produce the work However: with > >0 heat pump heat engine X Th C T T c c We can design the engine X such that

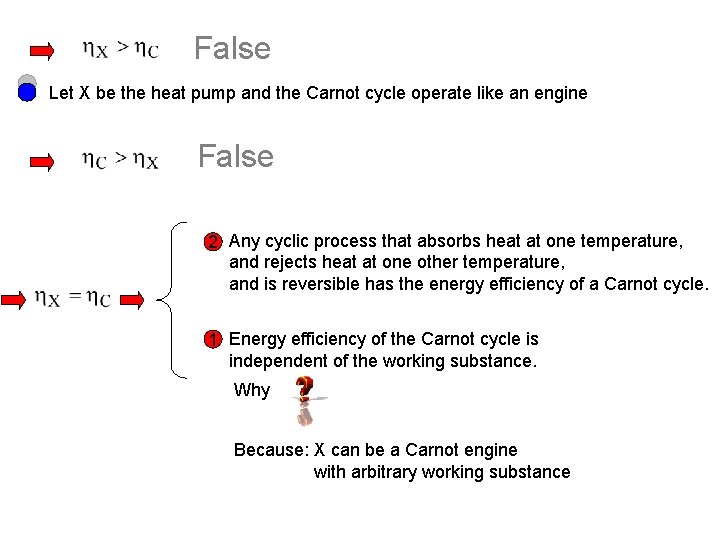
False Let X be the heat pump and the Carnot cycle operate like an engine False 2 Any cyclic process that absorbs heat at one temperature, and rejects heat at one other temperature, and is reversible has the energy efficiency of a Carnot cycle. 1 Energy efficiency of the Carnot cycle is independent of the working substance. Why Because: X can be a Carnot engine with arbitrary working substance

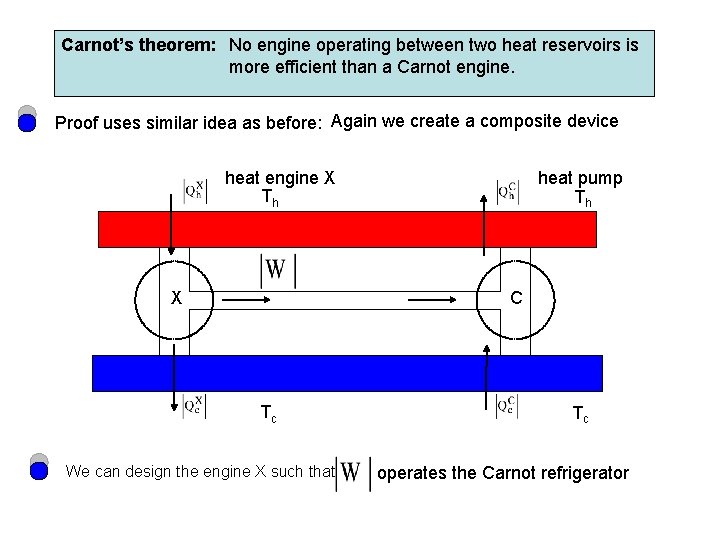
Carnot’s theorem: No engine operating between two heat reservoirs is more efficient than a Carnot engine. Proof uses similar idea as before: Again we create a composite device heat engine X Th X heat pump Th C Tc We can design the engine X such that Tc operates the Carnot refrigerator

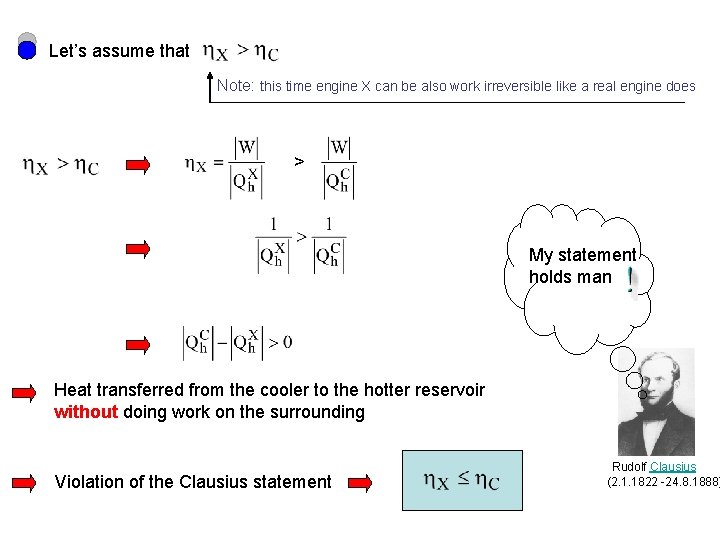
Let’s assume that Note: this time engine X can be also work irreversible like a real engine does > My statement holds man Heat transferred from the cooler to the hotter reservoir without doing work on the surrounding Violation of the Clausius statement Rudolf Clausius (2. 1. 1822 -24. 8. 1888)

Applications of Carnot Cycles We stated: Any cyclic process that absorbs heat at one temperature, and rejects heat at one other temperature, and is reversible has the energy efficiency of a Carnot cycle. - gas turbine Why did we calculate energy efficiencies for - Otto cycle Because: they are not 2 -temperature devices, but accept and reject heat at a range of temperatures Energy efficiency not given by the Carnot formula But: It is interesting to compare the maximum possible efficiency of a Carnot cycle with the efficiency of engineering cycles with the same maximum and minimum temperatures

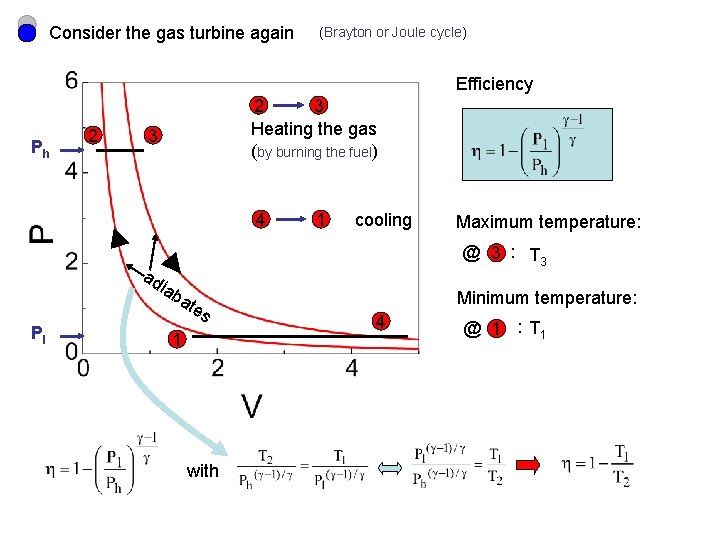
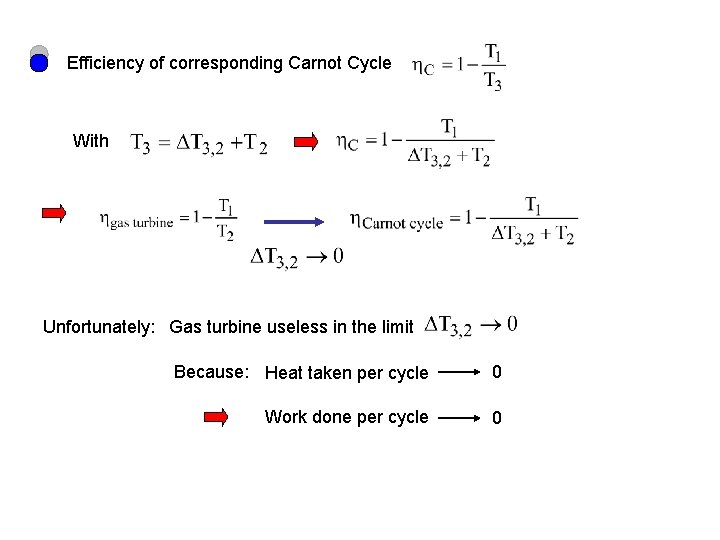
Consider the gas turbine again (Brayton or Joule cycle) Efficiency Ph 2 2 3 Heating the gas (by burning the fuel) 3 4 cooling Maximum temperature: @ 3 : T 3 ad iab Pl 1 ate s 1 with Minimum temperature: 4 @ 1 : T 1

Efficiency of corresponding Carnot Cycle With Unfortunately: Gas turbine useless in the limit Because: Heat taken per cycle 0 Work done per cycle 0

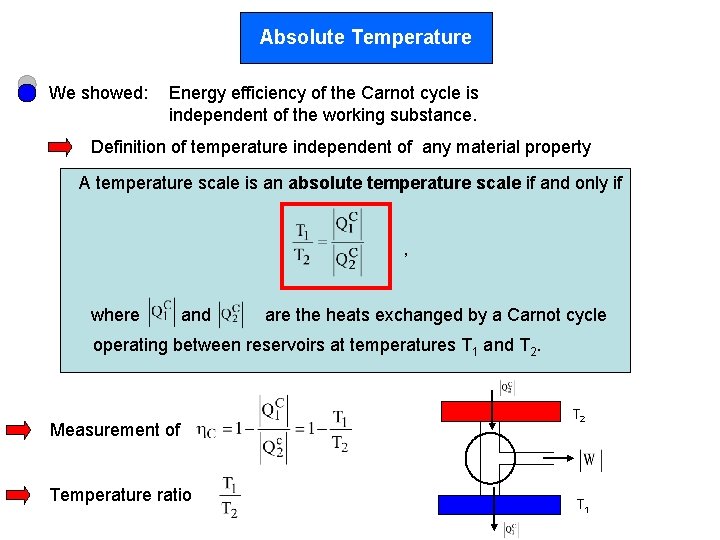
Absolute Temperature We showed: Energy efficiency of the Carnot cycle is independent of the working substance. Definition of temperature independent of any material property A temperature scale is an absolute temperature scale if and only if , where and are the heats exchanged by a Carnot cycle operating between reservoirs at temperatures T 1 and T 2. Measurement of Temperature ratio T 2 T 1

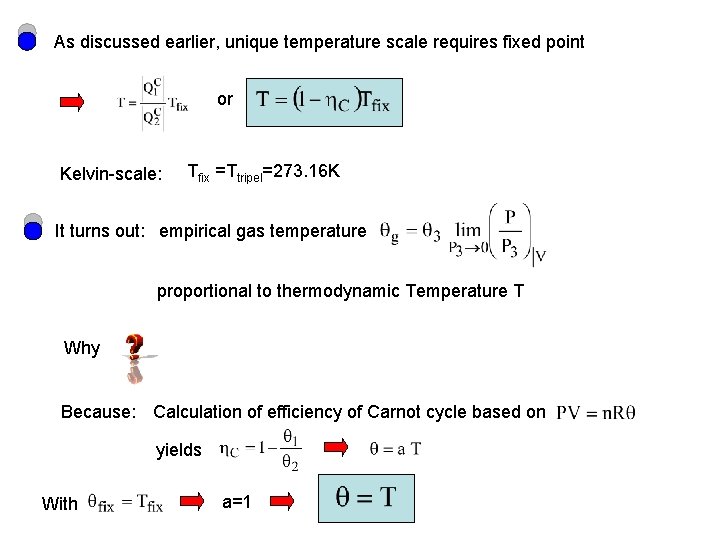
As discussed earlier, unique temperature scale requires fixed point or Kelvin-scale: Tfix =Ttripel=273. 16 K It turns out: empirical gas temperature proportional to thermodynamic Temperature T Why Because: Calculation of efficiency of Carnot cycle based on yields With a=1

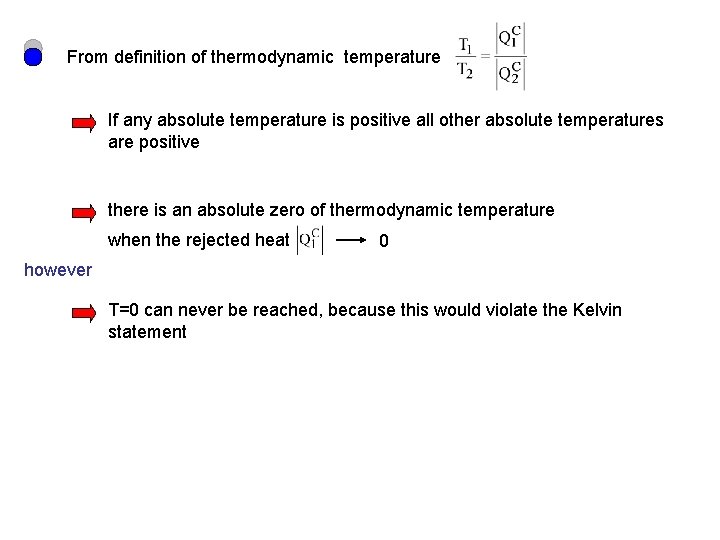
From definition of thermodynamic temperature If any absolute temperature is positive all other absolute temperatures are positive there is an absolute zero of thermodynamic temperature when the rejected heat 0 however T=0 can never be reached, because this would violate the Kelvin statement