CARNOT CYCLE I am teaching Engineering Thermodynamics using




- Slides: 28

CARNOT CYCLE I am teaching Engineering Thermodynamics using the textbook by Cengel and Boles. This set of slides overlap somewhat with Chapter 6. But here I assume that we have established the concept of entropy, and use the concept to analyze the Carnot cycle in the same way as we analyze any othermodynamic process. An isolated system conserves energy and generates entropy. I did add a few slides to show Carnot motivated his idea of entropy using the analogy of waterfall. I used the Dover edition of his book. I went through these slides in one 90 -minute lecture. Zhigang Suo, Harvard University

Thermodynamics relates heat and motion thermo = heat dynamics = motion

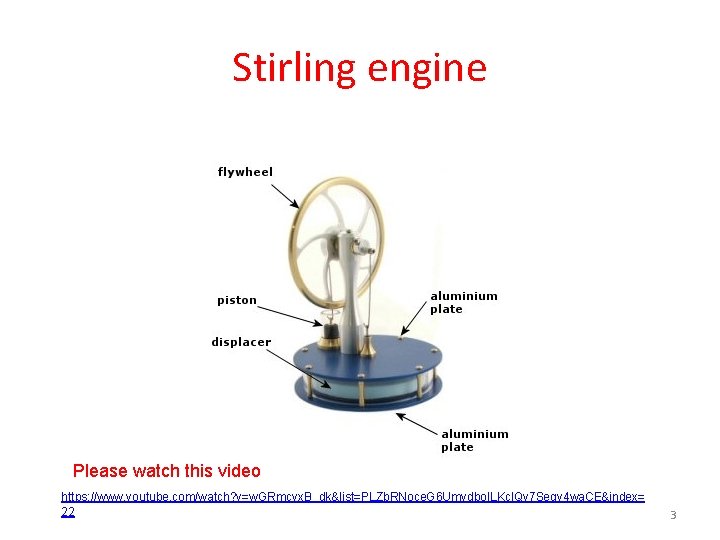
Stirling engine Please watch this video https: //www. youtube. com/watch? v=w. GRmcvx. B_dk&list=PLZb. RNoce. G 6 Umydbo. ILKcl. Qv 7 Seqy 4 wa. CE&index= 22 3

Carnot’s question How much work can be produced from a given quantity of heat? “…whether the motive power of heat is unbounded, whether the possible improvements in steam-engines have an assignable limit, a limit which the nature of things will not allow to be passed by any means whatever. . . ” Modern translations Motive power: work Motive power of heat: work produced by heat Limit: Carnot limit, Carnot efficiency Carnot, Reflections on the Motive Power of Fire (1824) 4

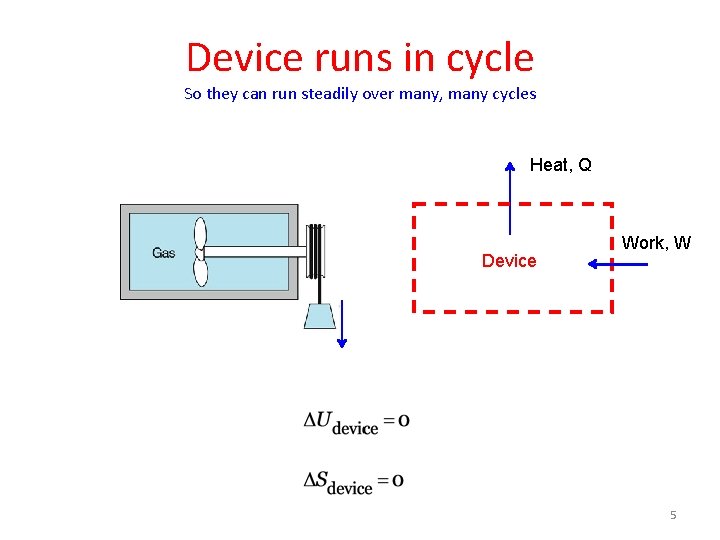
Device runs in cycle So they can run steadily over many, many cycles Heat, Q Device Work, W 5

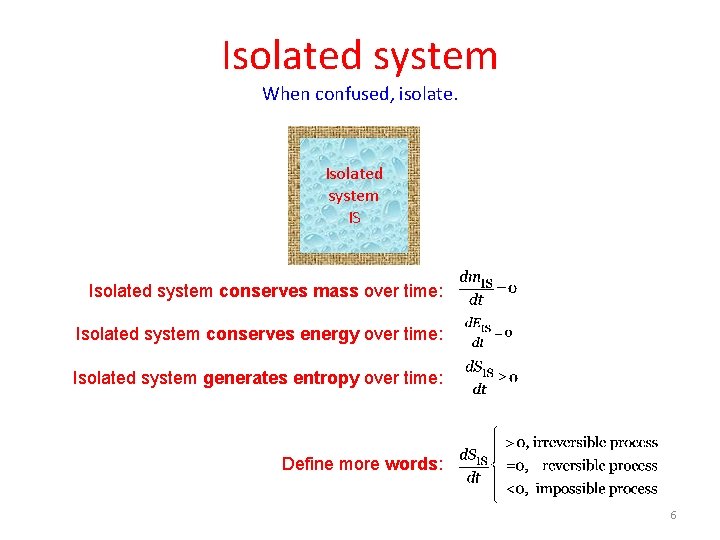
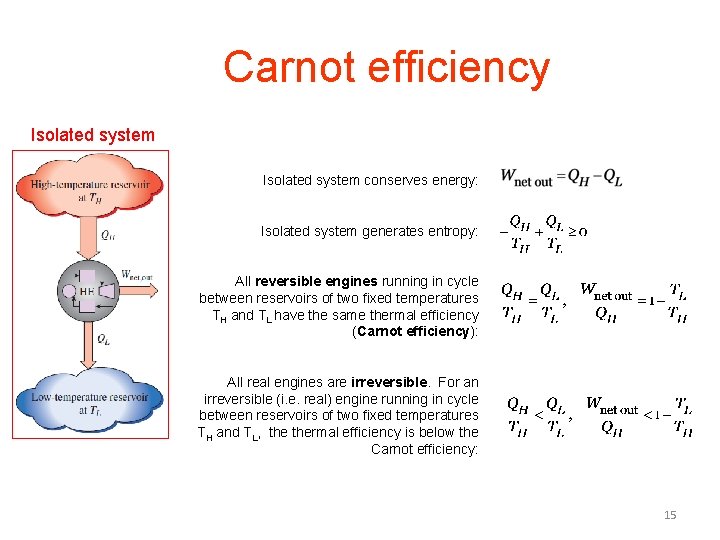
Isolated system When confused, isolate. Isolated system IS Isolated system conserves mass over time: Isolated system conserves energy over time: Isolated system generates entropy over time: Define more words: 6

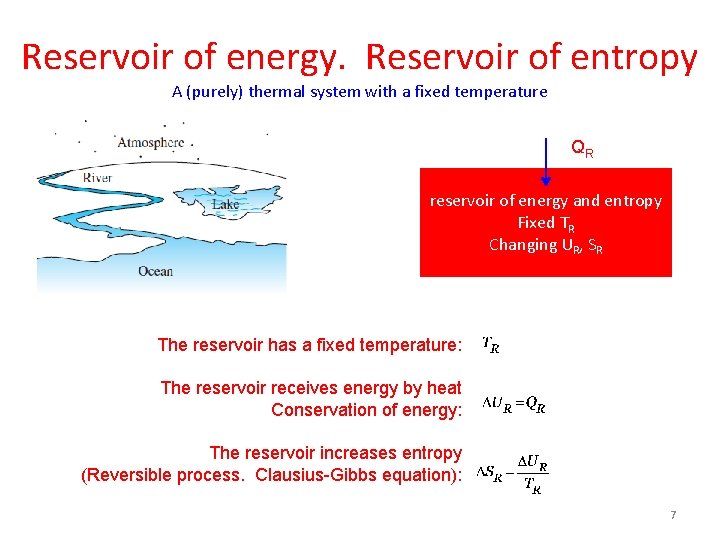
Reservoir of energy. Reservoir of entropy A (purely) thermal system with a fixed temperature QR reservoir of energy and entropy Fixed TR Changing UR, SR The reservoir has a fixed temperature: The reservoir receives energy by heat Conservation of energy: The reservoir increases entropy (Reversible process. Clausius-Gibbs equation): 7

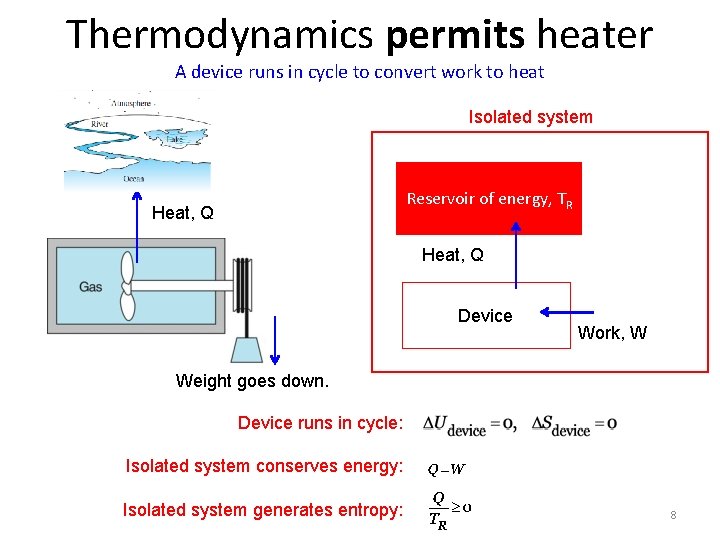
Thermodynamics permits heater A device runs in cycle to convert work to heat Isolated system Reservoir of energy, TR Heat, Q Device Work, W Weight goes down. Device runs in cycle: Isolated system conserves energy: Isolated system generates entropy: 8

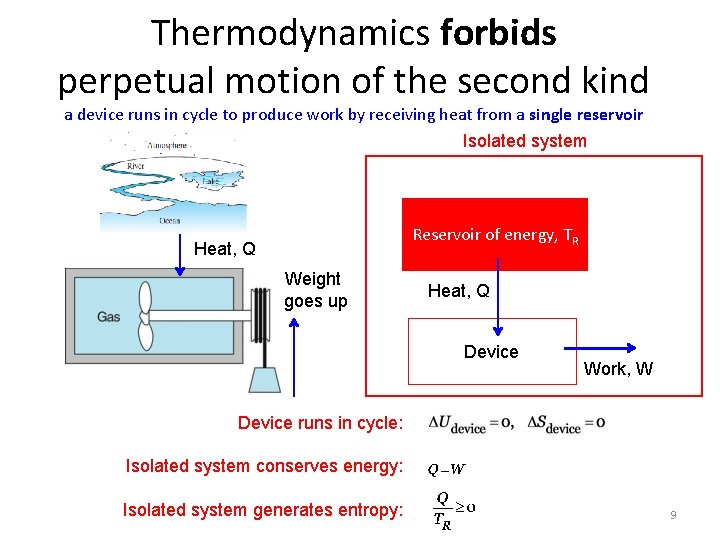
Thermodynamics forbids perpetual motion of the second kind a device runs in cycle to produce work by receiving heat from a single reservoir Isolated system Reservoir of energy, TR Heat, Q Weight goes up Heat, Q Device Work, W Device runs in cycle: Isolated system conserves energy: Isolated system generates entropy: 9

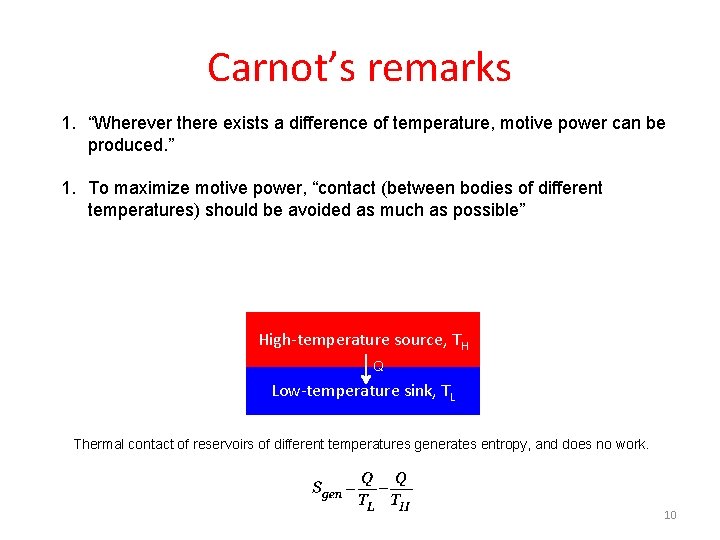
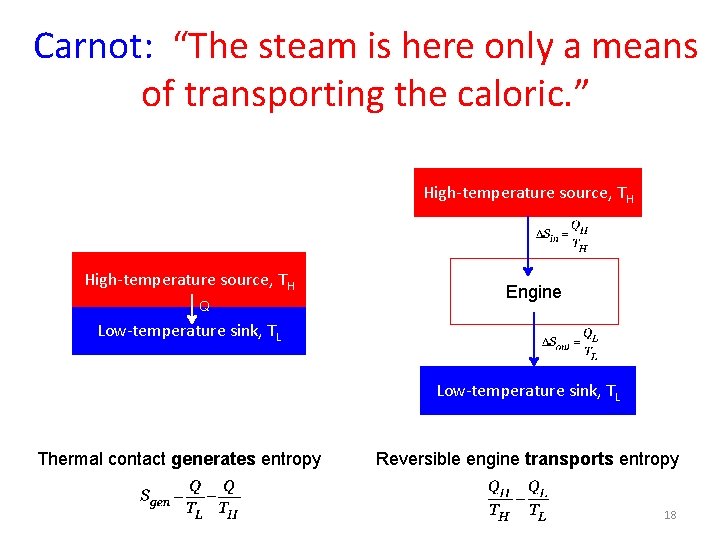
Carnot’s remarks 1. “Wherever there exists a difference of temperature, motive power can be produced. ” 1. To maximize motive power, “contact (between bodies of different temperatures) should be avoided as much as possible” High-temperature source, TH Q Low-temperature sink, TL Thermal contact of reservoirs of different temperatures generates entropy, and does no work. 10

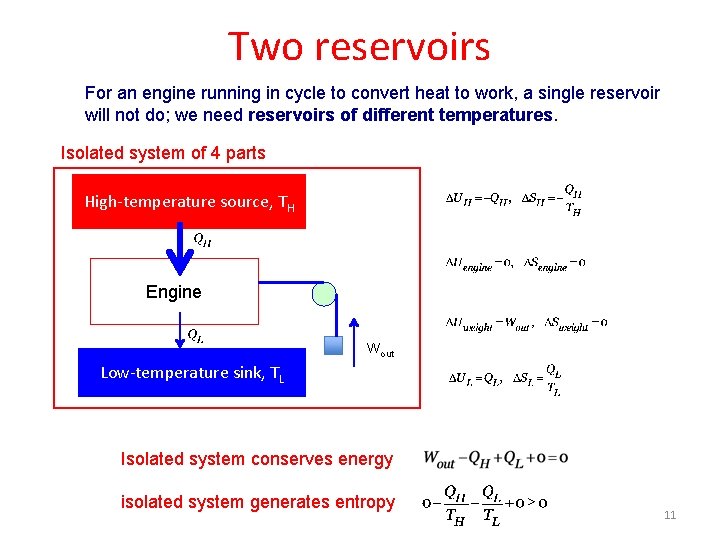
Two reservoirs For an engine running in cycle to convert heat to work, a single reservoir will not do; we need reservoirs of different temperatures. Isolated system of 4 parts High-temperature source, TH Engine Wout Low-temperature sink, TL Isolated system conserves energy isolated system generates entropy 11

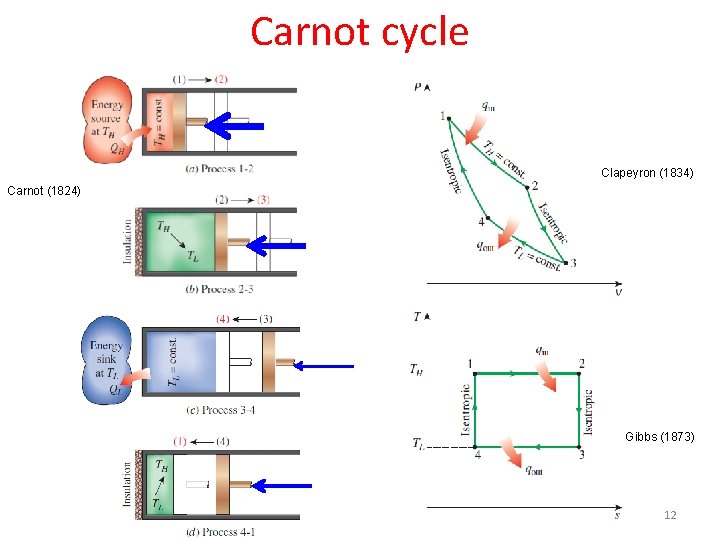
Carnot cycle Clapeyron (1834) Carnot (1824) Gibbs (1873) 12

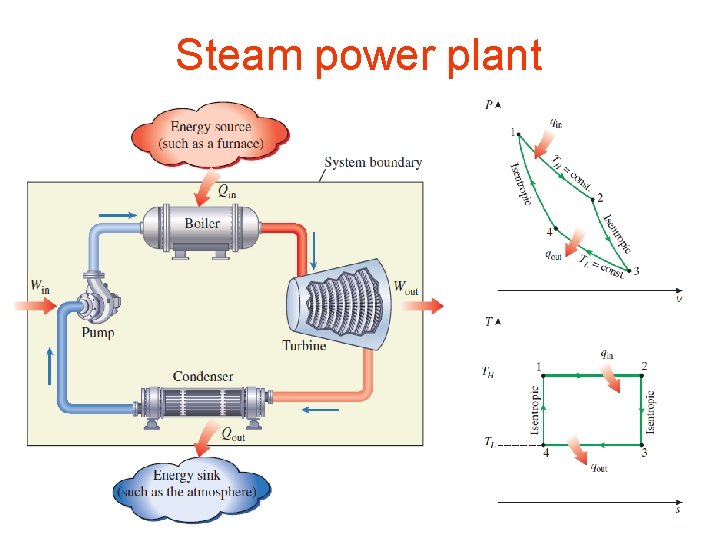
Steam power plant 13

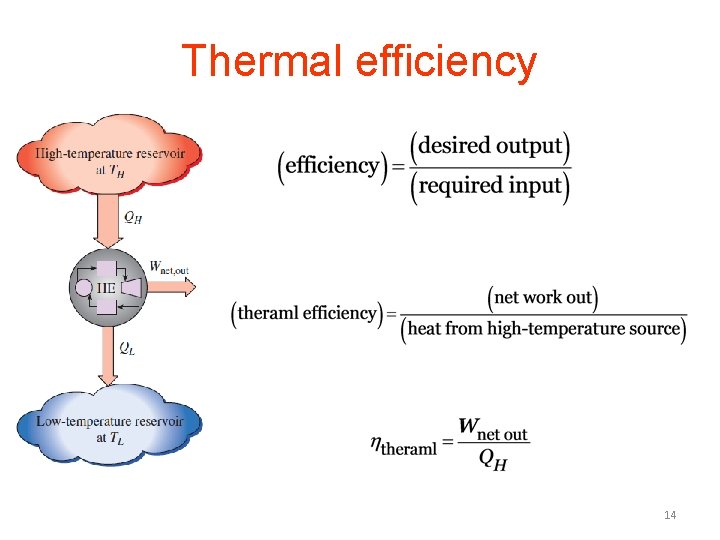
Thermal efficiency 14

Carnot efficiency Isolated system conserves energy: Isolated system generates entropy: All reversible engines running in cycle between reservoirs of two fixed temperatures TH and TL have the same thermal efficiency (Carnot efficiency): All real engines are irreversible. For an irreversible (i. e. real) engine running in cycle between reservoirs of two fixed temperatures TH and TL, thermal efficiency is below the Carnot efficiency: 15

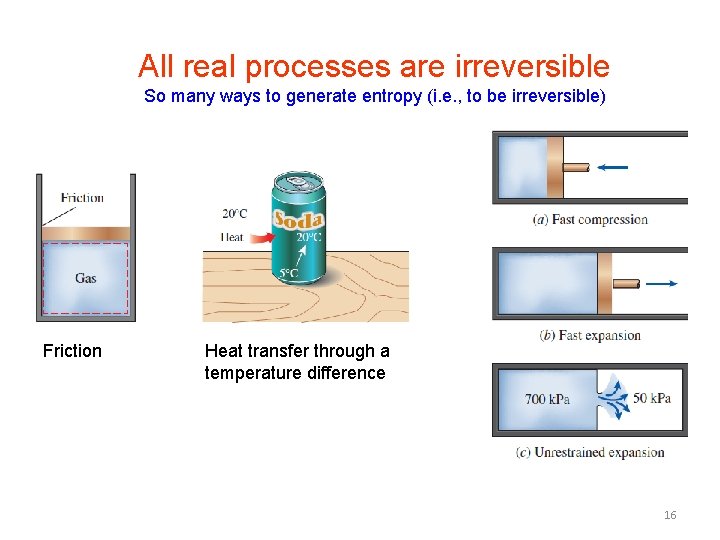
All real processes are irreversible So many ways to generate entropy (i. e. , to be irreversible) Friction Heat transfer through a temperature difference 16

Carnot (1824): Two reservoirs Reflections on the Motive Power of Fire. …the re-establishing of equilibrium in the caloric; that is, its passage from a body in which the temperature is more or less elevated, to another in which it is lower. What happens in fact in a steam-engine actually in motion? The caloric developed in the furnace by the effect of the combustion traverses the walls of the boiler, produces steam, and in some way incorporates itself with it. The latter carrying it away, takes it first into the cylinder, where it performs some function, and from thence into the condenser, where it is liquefied by contact with the cold water which it encounters there. Then, as a final result, the cold water of the condenser takes possession of the caloric developed by the combustion. . . The steam is here only a means of transporting the caloric. These two bodies, to which we can give or from which we can remove the heat without causing their temperatures to vary, exercise the functions of two unlimited reservoirs of caloric. Modern translation Caloric: entropy Reservoir of caloric: Thermal reservoir Carnot (1796 -1832) 17

Carnot: “The steam is here only a means of transporting the caloric. ” High-temperature source, TH Q Engine Low-temperature sink, TL Thermal contact generates entropy Reversible engine transports entropy 18

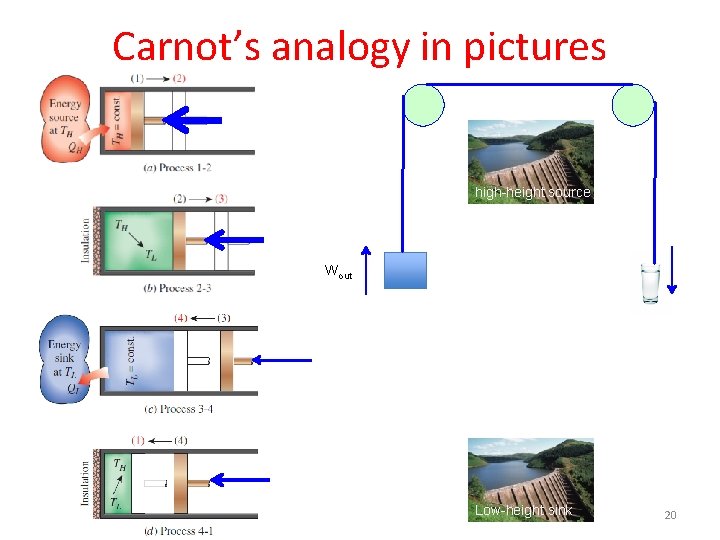
Carnot’s analogy in his own words The motive power of a waterfall depends on its height and on the quantity of the liquid; the motive power of heat depends also on the quantity of caloric used, and on what may be termed, on what in fact we will call, the height of its fall, that is to say, the difference of temperature of the bodies between which the exchange of caloric is made. In the waterfall the motive power is exactly proportional to the difference of level between the higher and lower reservoirs. In the fall of caloric the motive power undoubtedly increases with the difference of temperature between the warm and the cold bodies; but we do not know whether it is proportional to this difference. We do not know, for example, whether the fall of caloric from 100 to 50 degrees furnishes more or less motive power than the fall of this same caloric from 50 to zero. It is a question which we propose to examine hereafter. Modern translations Motive power: work Caloric: entropy Carnot, Reflections on the Motive Power of Fire (1824) 19

Carnot’s analogy in pictures high-height source Wout Low-height sink 20

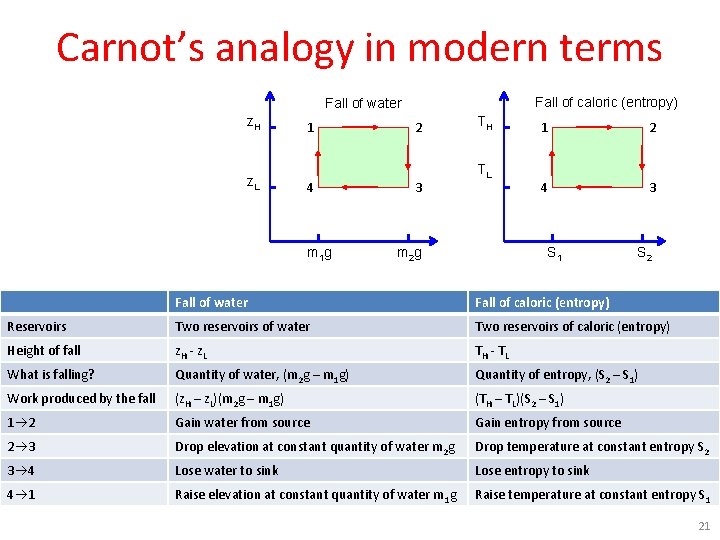
Carnot’s analogy in modern terms Fall of caloric (entropy) Fall of water z. H z. L 1 4 m 1 g 2 3 TH TL m 2 g 1 2 4 3 S 1 S 2 Fall of water Fall of caloric (entropy) Reservoirs Two reservoirs of water Two reservoirs of caloric (entropy) Height of fall z. H - z. L TH - T L What is falling? Quantity of water, (m 2 g – m 1 g) Quantity of entropy, (S 2 – S 1) Work produced by the fall (z. H – z. L)(m 2 g – m 1 g) (TH – TL)(S 2 – S 1) 1 2 Gain water from source Gain entropy from source 2 3 Drop elevation at constant quantity of water m 2 g Drop temperature at constant entropy S 2 3 4 Lose water to sink Lose entropy to sink 4 1 Raise elevation at constant quantity of water m 1 g Raise temperature at constant entropy S 1 21

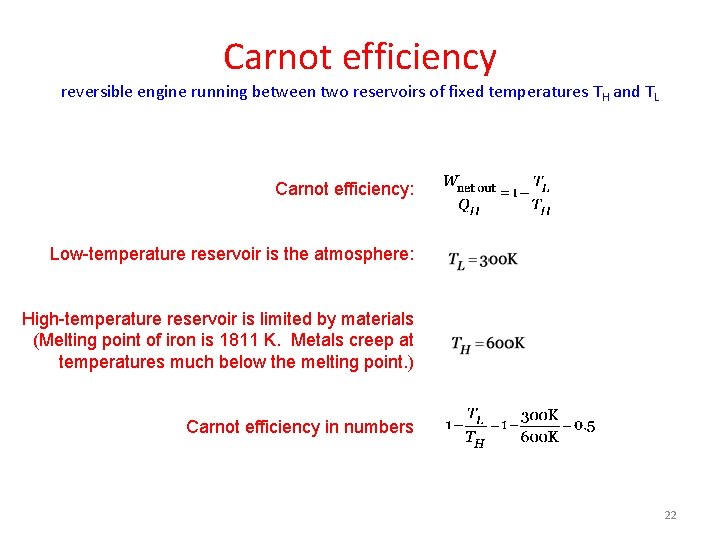
Carnot efficiency reversible engine running between two reservoirs of fixed temperatures T H and TL Carnot efficiency: Low-temperature reservoir is the atmosphere: High-temperature reservoir is limited by materials (Melting point of iron is 1811 K. Metals creep at temperatures much below the melting point. ) Carnot efficiency in numbers 22

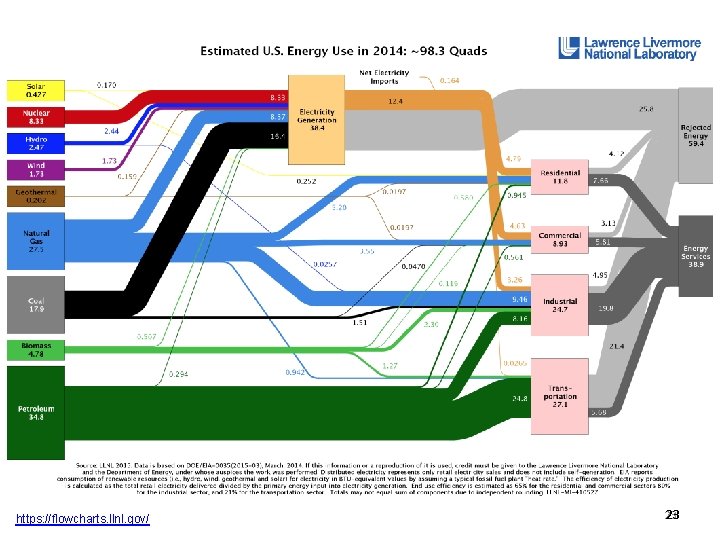
https: //flowcharts. llnl. gov/ 23 23

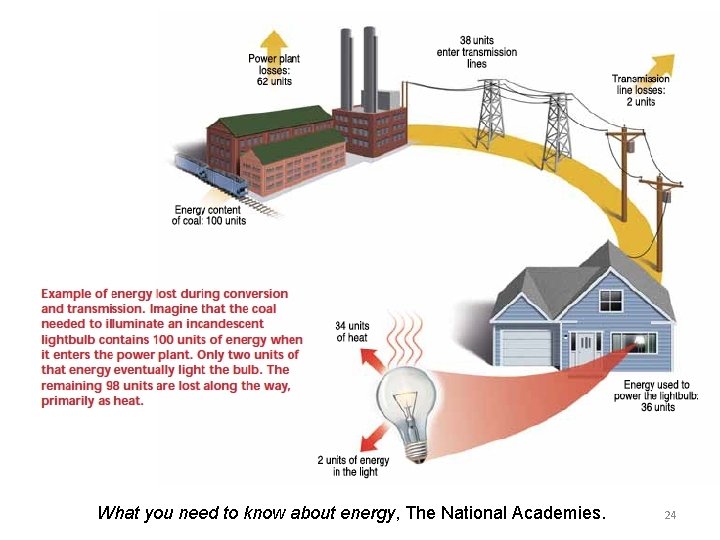
What you need to know about energy, The National Academies. 24

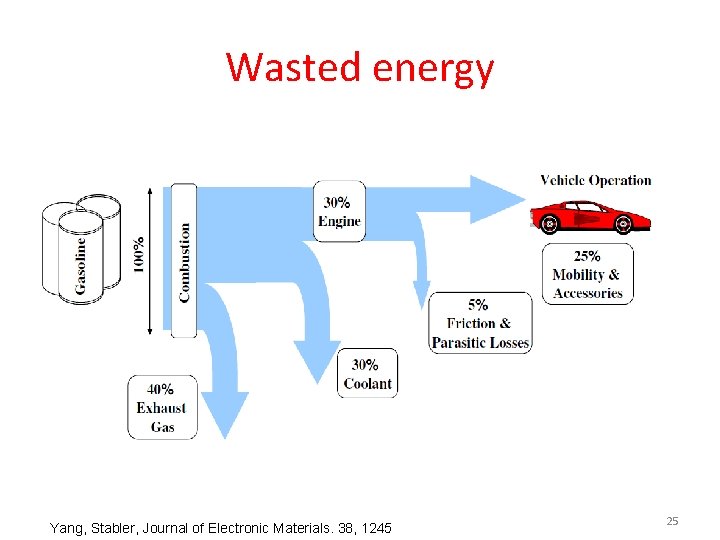
Wasted energy Yang, Stabler, Journal of Electronic Materials. 38, 1245 25

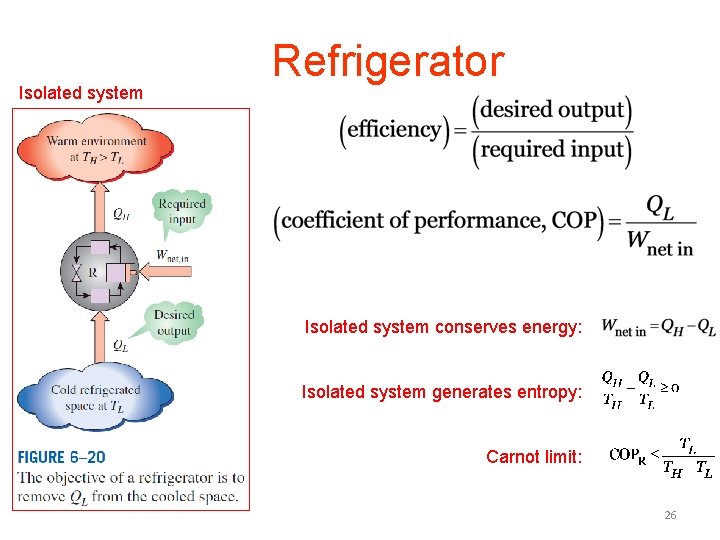
Isolated system Refrigerator Isolated system conserves energy: Isolated system generates entropy: Carnot limit: 26

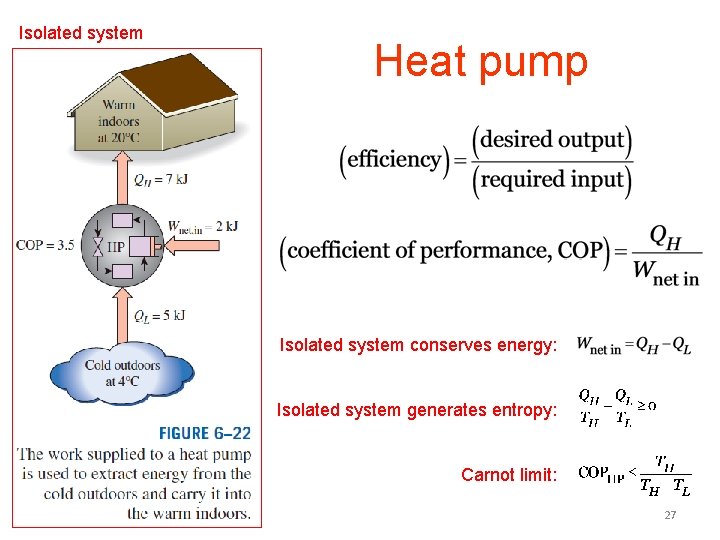
Isolated system Heat pump Isolated system conserves energy: Isolated system generates entropy: Carnot limit: 27

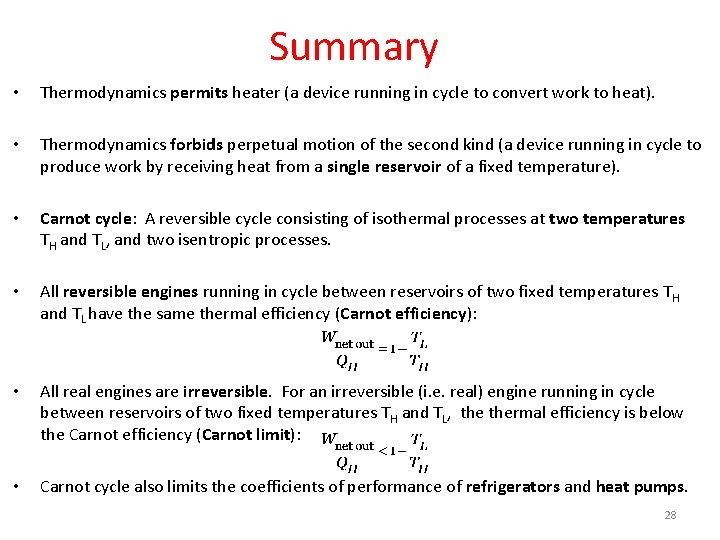
Summary • Thermodynamics permits heater (a device running in cycle to convert work to heat). • Thermodynamics forbids perpetual motion of the second kind (a device running in cycle to produce work by receiving heat from a single reservoir of a fixed temperature). • Carnot cycle: A reversible cycle consisting of isothermal processes at two temperatures TH and TL, and two isentropic processes. • All reversible engines running in cycle between reservoirs of two fixed temperatures TH and TL have the same thermal efficiency (Carnot efficiency): • All real engines are irreversible. For an irreversible (i. e. real) engine running in cycle between reservoirs of two fixed temperatures TH and TL, thermal efficiency is below the Carnot efficiency (Carnot limit): • Carnot cycle also limits the coefficients of performance of refrigerators and heat pumps. 28