Cardio Vasculaire Genees Kunde nl SPIRE Program Studies



- Slides: 10

Cardio. Vasculaire. Genees. Kunde. nl SPIRE Program: Studies of PCSK 9 Inhibition and the Reduction of Vascular Events Unanticipated attenuation of LDL-c lowering response to humanized PCSK 9 antibody over time Ridker PM, Tardif J-C, Amarenco P, et al. Lipid-Reduction Variability and Antidrug-Antibody Formation with Bococizumab. NEJM 2017; 376: 1517 -1526 Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular Efficacy and Safety of Bococizumab in High-Risk Patients. NEJM 2017; 376: 1527 -1539

SPIRE: Background and Objective Background In six multinational trials evaluating bococizumab, a PCSK 9 inhibitor, the initially achieved LDL-c lowering was significantly attenuated over time due to antidrug antibodies, and the relative reduction in cholesterol levels varied widely also in patients without antidrug antibody development. Study Objective The SPIRE-1 and SPIRE-2 studies were designed to assess the clinical efficacy and safety of bococizumab versus placebo in patients at high CV risk with 2 different baseline levels of LDL-c. When the results of the six SPIRE lipid-lowering trials became available, the sponsor discontinued prematurely the ongoing SPIRE-1 and SPIRE-2 outcome trials. PCSK 9: proprotein convertase subtilisin–kexin type 9; LDL-c: low density lipoprotein cholesterol; CV: cardiovascular Ridker PM, et al. , N Engl J Med 2017; 376: 1517 -1526 Ridker PM, et al. , N Engl J Med 2017; 376: 1527 -1539

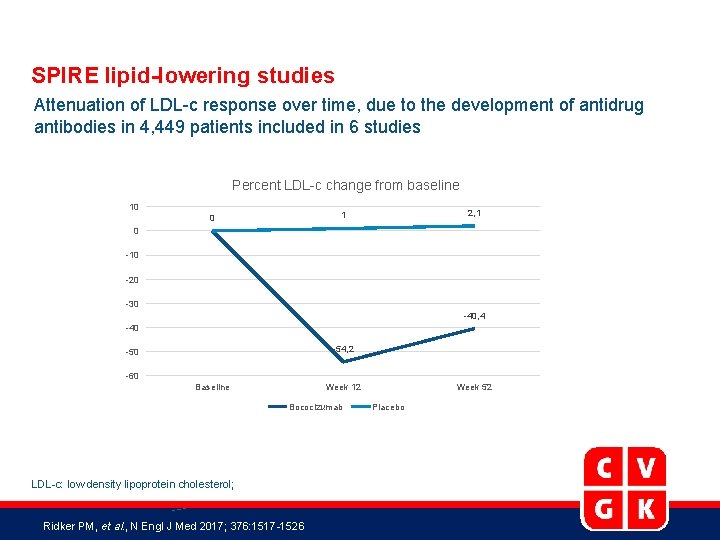
SPIRE lipid-lowering studies Attenuation of LDL-c response over time, due to the development of antidrug antibodies in 4, 449 patients included in 6 studies Percent LDL-c change from baseline 10 2, 1 1 0 0 -10 -20 -30 -40, 4 -40 -54, 2 -50 -60 Baseline Week 12 Bococizumab LDL-c: low density lipoprotein cholesterol; Ridker PM, et al. , N Engl J Med 2017; 376: 1517 -1526 Week 52 Placebo

SPIRE-1 & -2: Inclusion criteria in both studies • • Secondary prevention cohort: history of CV event, or IS, or coronary artery or peripheral artery revascularization High-risk primary prevention cohort: history of DM, or KD, or PVD plus at least 1 CV risk factor* or history of FHC Lipid levels inclusion criteria • • SPIRE-1: LDL-c ≥ 70 mg/d. L (1. 81 mmol/L) or non-HDL-c ≥ 100 mg/d. L (2. 59 mmol/L) SPIRE-2: LDL-c ≥ 100 mg/d. L (2. 59 mmol/L) or non-HDL-c ≥ 130 mg/d. L (3. 36 mmol/L) (*) smoking history, HDL-c <40 mg/d. L (<1. 0 mmol/L), hs-CRP >2. 0 mg/L; Lp(a) > 50 mg/d. L, microalbuminuria, asymptomatic coronary stenosis; age ≥ 50 years for men and ≥ 60 years for women CV: cardiovascular; IS: ischemic stroke; DM; diabetes mellitus; KD: kidney disease; PVD: peripheral vascular disease; FHC: familial hypercholesterolemia; LDL-c: low density lipoprotein cholesterol; HDL-c: high density lipoprotein cholesterol; hs-CRP: high sensitivity Creactive protein; Lp(a): lipoprotein(a); Ridker PM, et al. , N Engl J Med 2017; 376: 1527 -1539

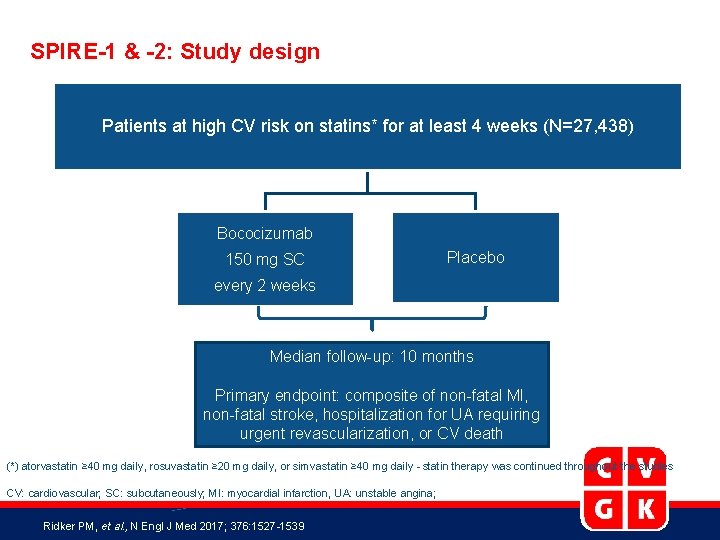
SPIRE-1 & -2: Study design Patients at high CV risk on statins* for at least 4 weeks (N=27, 438) Bococizumab 150 mg SC Placebo every 2 weeks Median follow-up: 10 months Primary endpoint: composite of non-fatal MI, non-fatal stroke, hospitalization for UA requiring urgent revascularization, or CV death (*) atorvastatin ≥ 40 mg daily, rosuvastatin ≥ 20 mg daily, or simvastatin ≥ 40 mg daily - statin therapy was continued throughout the studies CV: cardiovascular; SC: subcutaneously; MI: myocardial infarction, UA: unstable angina; Ridker PM, et al. , N Engl J Med 2017; 376: 1527 -1539

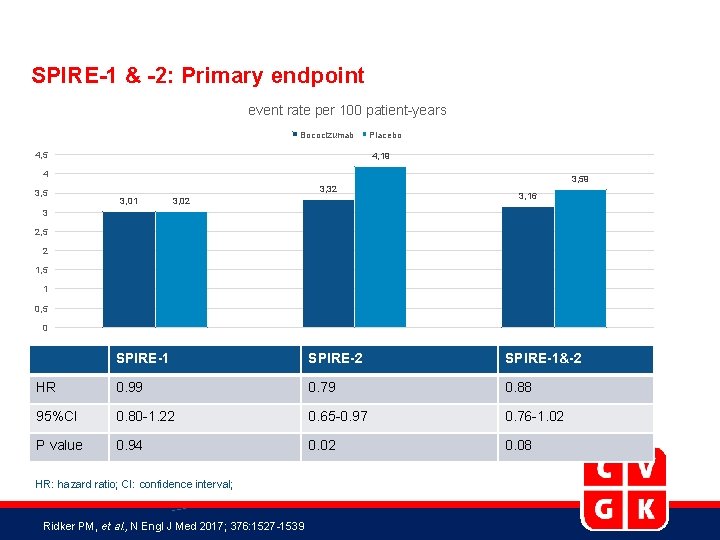
SPIRE-1 & -2: Primary endpoint event rate per 100 patient-years Bococizumab 4, 5 Placebo 4, 19 4 3, 59 3, 32 3, 01 3, 02 3, 16 3 2, 5 2 1, 5 1 0, 5 0 SPIRE-1 SPIRE-2 SPIRE-1&-2 HR 0. 99 0. 79 0. 88 95%CI 0. 80 -1. 22 0. 65 -0. 97 0. 76 -1. 02 P value 0. 94 0. 02 0. 08 HR: hazard ratio; CI: confidence interval; Ridker PM, et al. , N Engl J Med 2017; 376: 1527 -1539

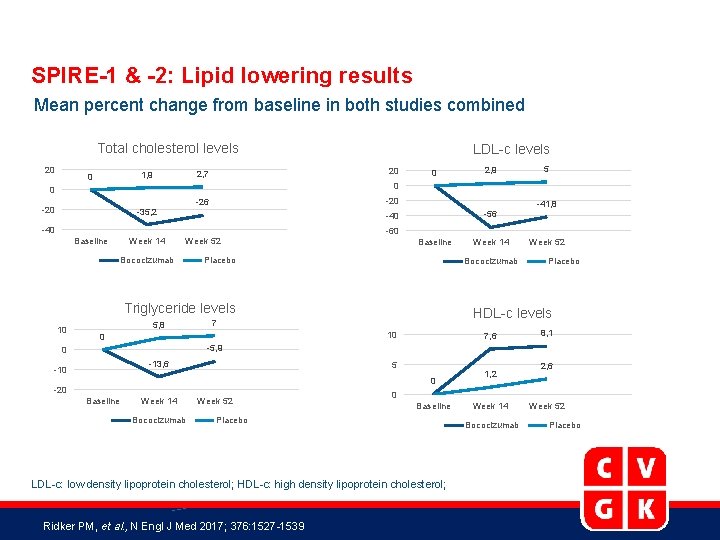
SPIRE-1 & -2: Lipid lowering results Mean percent change from baseline in both studies combined Total cholesterol levels 20 1, 9 0 LDL-c levels 2, 7 20 -26 -20 0 5 0 0 -20 -35, 2 Baseline Week 14 -41, 8 -56 -40 Week 52 Bococizumab -60 Baseline Placebo 5, 8 Week 52 Placebo HDL-c levels 7 10 0 Week 14 Bococizumab Triglyceride levels 10 2, 9 7, 6 8, 1 -5, 9 0 -13, 6 -10 5 0 -20 Baseline Week 14 Bococizumab Week 52 2, 6 0 Baseline Placebo LDL-c: low density lipoprotein cholesterol; HDL-c: high density lipoprotein cholesterol; Ridker PM, et al. , N Engl J Med 2017; 376: 1527 -1539 1, 2 Week 14 Bococizumab Week 52 Placebo

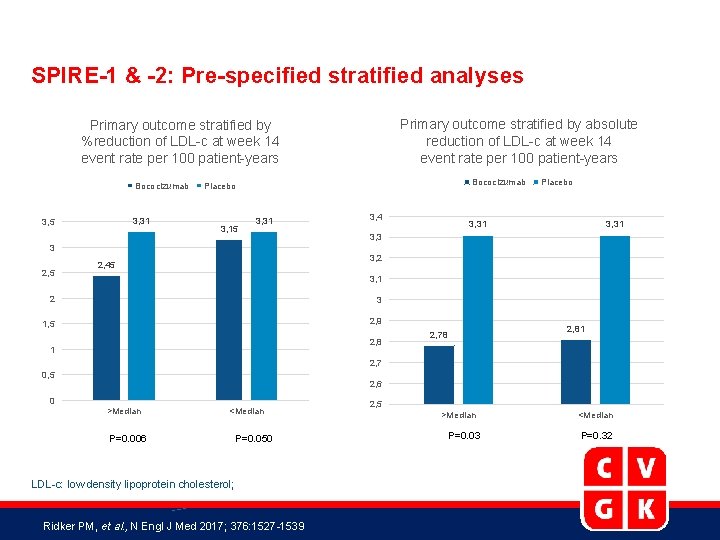
SPIRE-1 & -2: Pre-specified stratified analyses Primary outcome stratified by absolute reduction of LDL-c at week 14 event rate per 100 patient-years Primary outcome stratified by %reduction of LDL-c at week 14 event rate per 100 patient-years Bococizumab 3, 31 3, 5 Bococizumab Placebo 3, 15 3, 31 3, 4 Placebo 3, 31 3, 3 3 2, 5 3, 2 2, 45 3, 1 2 3 1, 5 2, 9 2, 8 1 2, 81 2, 78 2, 7 0, 5 2, 6 0 >Median <Median P=0. 006 P=0. 050 LDL-c: low density lipoprotein cholesterol; Ridker PM, et al. , N Engl J Med 2017; 376: 1527 -1539 2, 5 >Median P=0. 03 <Median P=0. 32

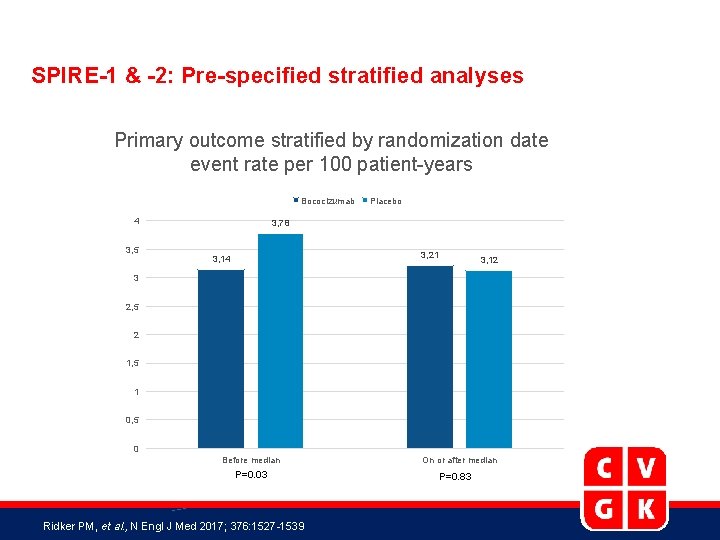
SPIRE-1 & -2: Pre-specified stratified analyses Primary outcome stratified by randomization date event rate per 100 patient-years Bococizumab 4 3, 5 Placebo 3, 78 3, 21 3, 14 3, 12 3 2, 5 2 1, 5 1 0, 5 0 Before median P=0. 03 Ridker PM, et al. , N Engl J Med 2017; 376: 1527 -1539 On or after median P=0. 83

SPIRE: Conclusions • PCSK 9 inhibition with bococizumab reduces LDL-c by 55 -60% when given as an adjunct to statin therapy, but this effect significantly attenuates over time due to the development of anti-drug antibodies. • Despite anti-drug antibody production and the early trial termination, bococizumab significantly reduced cardiovascular event rates in the higher-risk SPIRE-2 trial of those with LDL-c >100 mg/d. L, but not in the lower-risk SPIRE-1 trial of those with LDL-c >70 mg/d. L. • Clinical benefits were greater and statistically significant in analyses of those who achieved and sustained greater absolute and relative LDL-c reductions, and in those who were exposed to therapy for a longer time, a finding that supports the hypothesis that ‘lower is better for longer’. Ridker PM, et al. , N Engl J Med 2017; 376: 1527 -1539