Carboxylic Acids Esters Topic Chemistry Intro Carboxylic Acids
Carboxylic Acids & Esters
Topic Chemistry- Intro Carboxylic Acids and Esters Aims Ø To help introduce students to Carboxylic Acids and Esters Aimed at 16 -19 year olds Level 3 Method In-depth Power. Point slides, ALL can be hand-outs, some slides have questions and answers (could be done as a group discussion) Equipment Ø Ø Projector Laptop Pens Hand-Outs Duration >30 Mins

Learning Outcomes • 5 th functional group – Carboxylic Acids • 6 th Functional Group - Esters • Drawing Carboxylic Acids & Esters • Naming Carboxylic Acids & Esters • Physical properties of Carboxylic Acids & Esters • Chemical properties of Carboxylic Acids & Esters
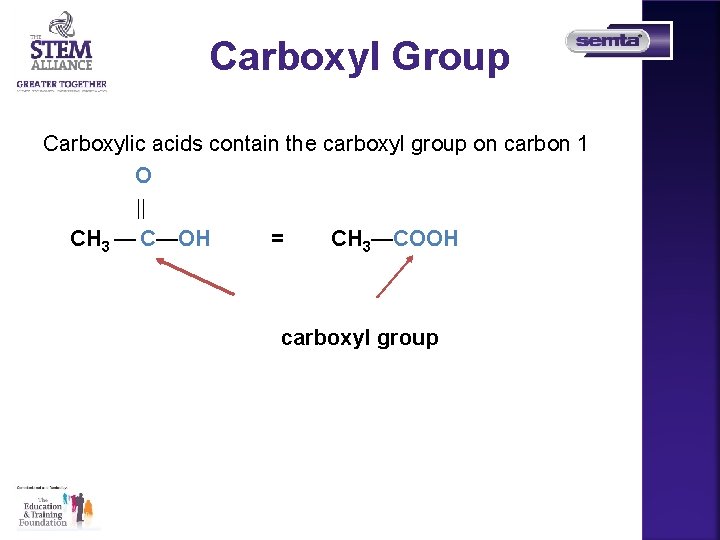
Carboxyl Group Carboxylic acids contain the carboxyl group on carbon 1 O CH 3 — C—OH = CH 3—COOH carboxyl group
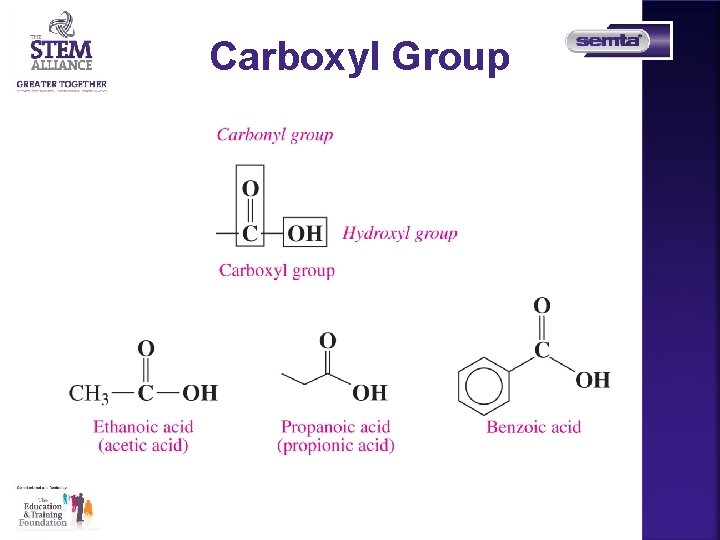
Carboxyl Group

Naming Carboxylic Acids
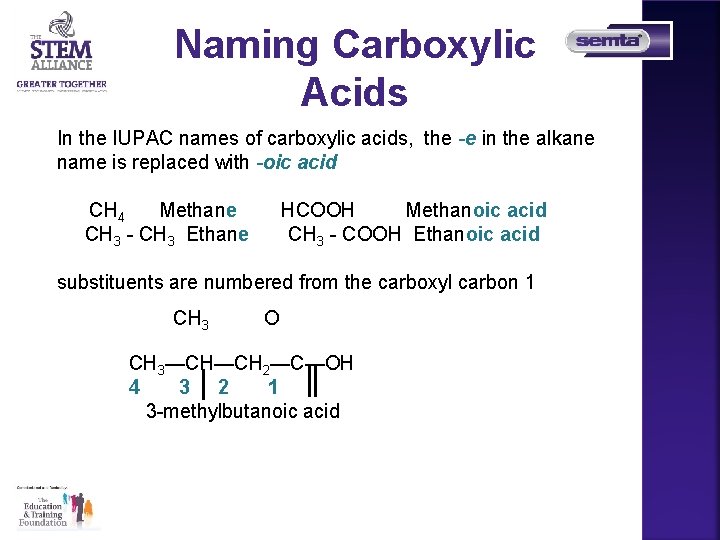
Naming Carboxylic Acids In the IUPAC names of carboxylic acids, the -e in the alkane name is replaced with -oic acid CH 4 Methane CH 3 - CH 3 Ethane HCOOH Methanoic acid CH 3 - COOH Ethanoic acid substituents are numbered from the carboxyl carbon 1 CH 3 O CH 3—CH—CH 2—C—OH 4 3 2 1 3 -methylbutanoic acid
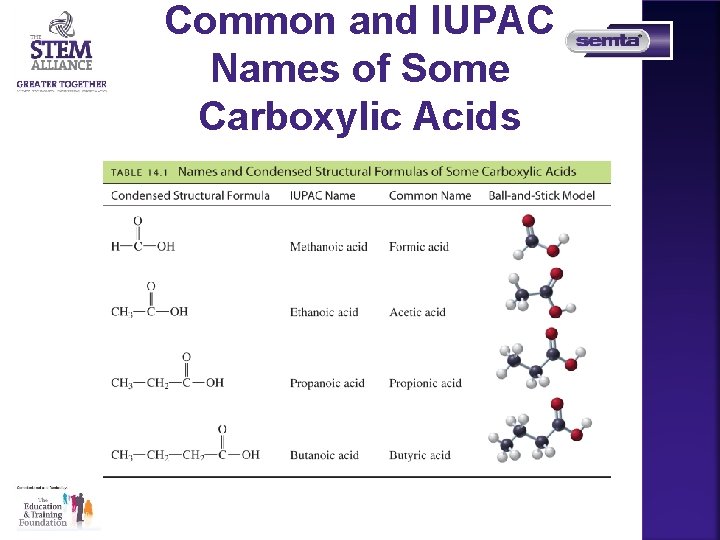
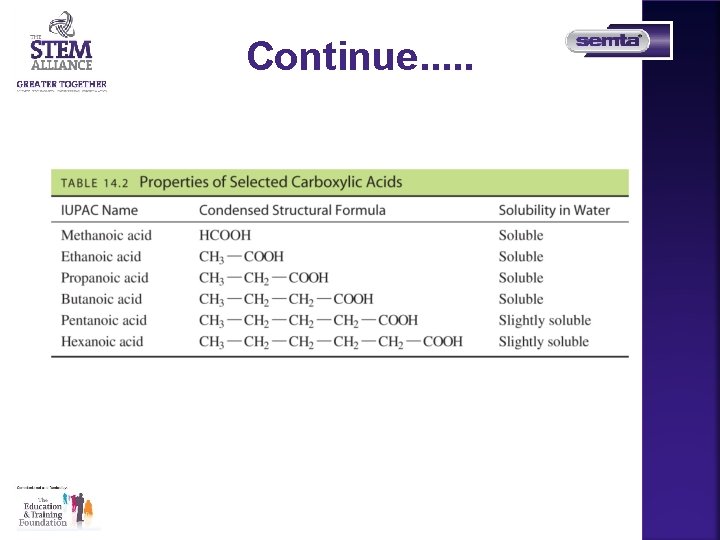
Common and IUPAC Names of Some Carboxylic Acids
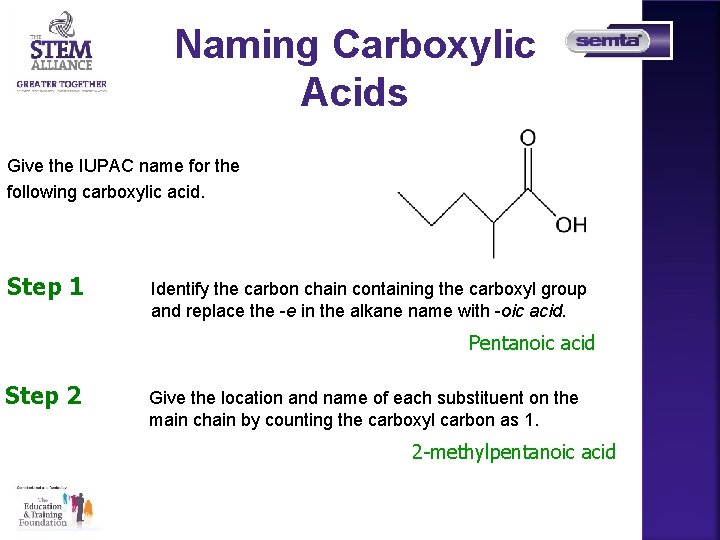
Naming Carboxylic Acids Give the IUPAC name for the following carboxylic acid. Step 1 Identify the carbon chain containing the carboxyl group and replace the -e in the alkane name with -oic acid. Pentanoic acid Step 2 Give the location and name of each substituent on the main chain by counting the carboxyl carbon as 1. 2 -methylpentanoic acid
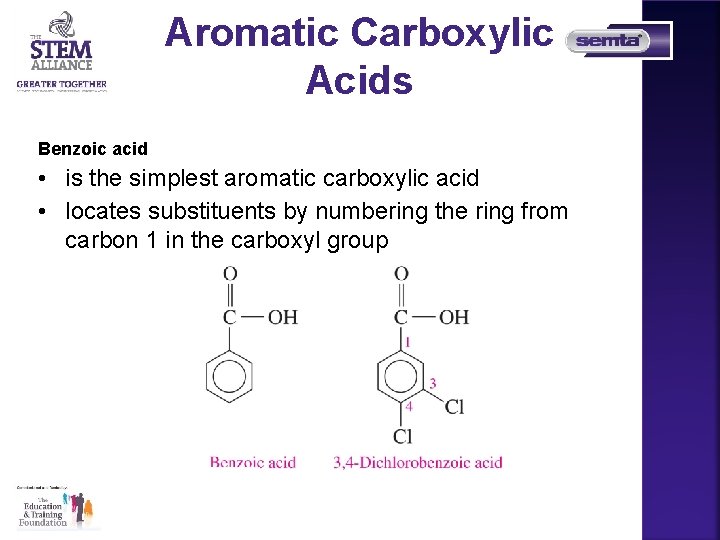
Aromatic Carboxylic Acids Benzoic acid • is the simplest aromatic carboxylic acid • locates substituents by numbering the ring from carbon 1 in the carboxyl group
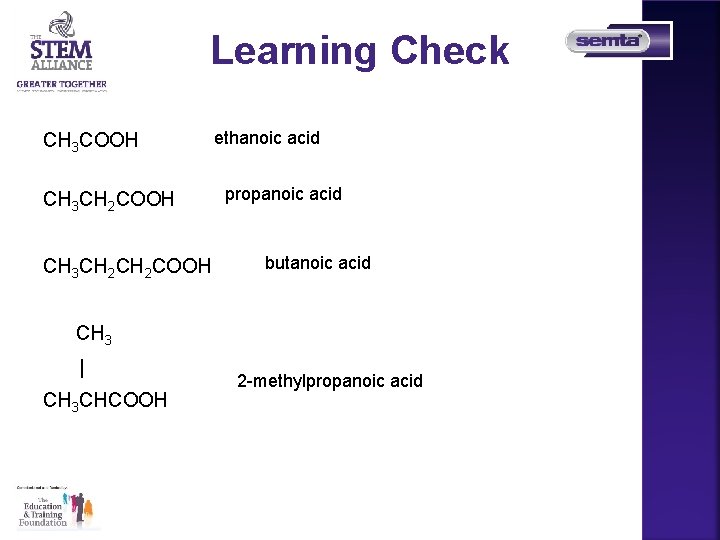
Learning Check CH 3 COOH CH 3 CH 2 CH 2 COOH ethanoic acid propanoic acid butanoic acid CH 3 | CH 3 CHCOOH 2 -methylpropanoic acid
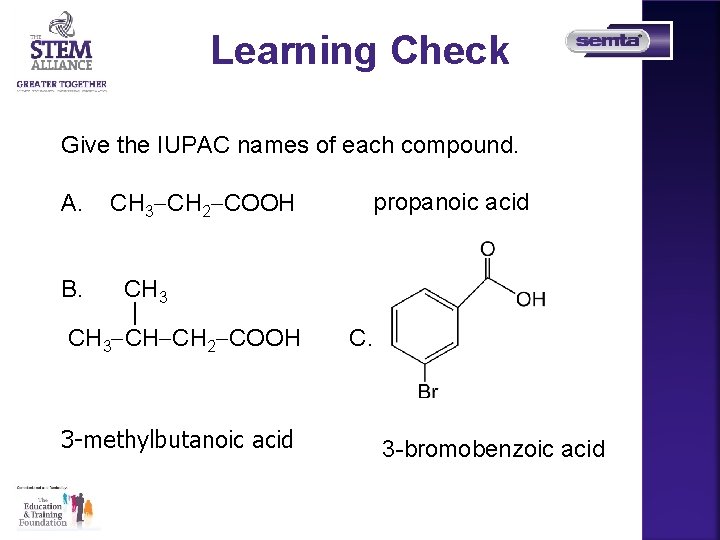
Learning Check Give the IUPAC names of each compound. A. CH 3 CH 2 COOH CH 3 | CH 3 CH CH 2 COOH propanoic acid B. 3 -methylbutanoic acid C. 3 -bromobenzoic acid
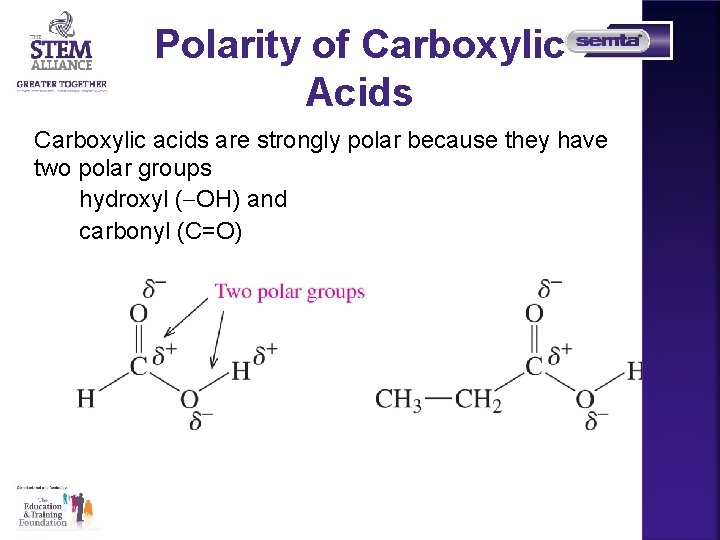
Polarity of Carboxylic Acids Carboxylic acids are strongly polar because they have two polar groups hydroxyl ( OH) and carbonyl (C=O)
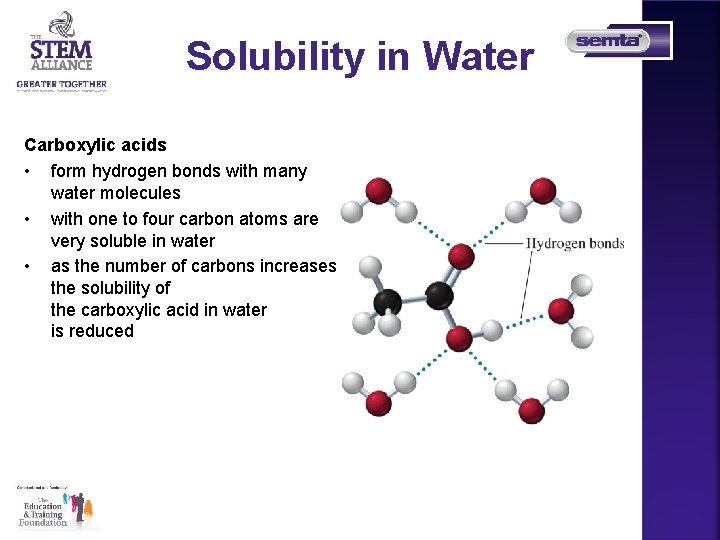
Solubility in Water Carboxylic acids • form hydrogen bonds with many water molecules • with one to four carbon atoms are very soluble in water • as the number of carbons increases, the solubility of the carboxylic acid in water is reduced
Continue. . .
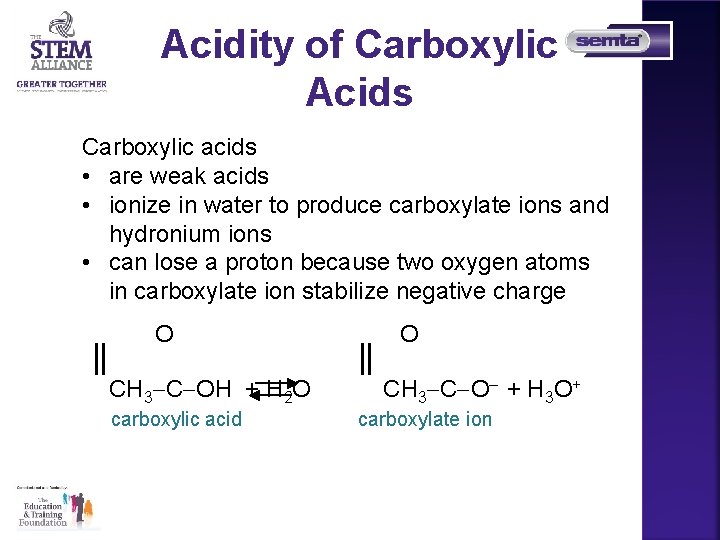
Acidity of Carboxylic Acids Carboxylic acids • are weak acids • ionize in water to produce carboxylate ions and hydronium ions • can lose a proton because two oxygen atoms in carboxylate ion stabilize negative charge O CH 3 C OH + H 2 O carboxylic acid O CH 3 C O– + H 3 O+ carboxylate ion
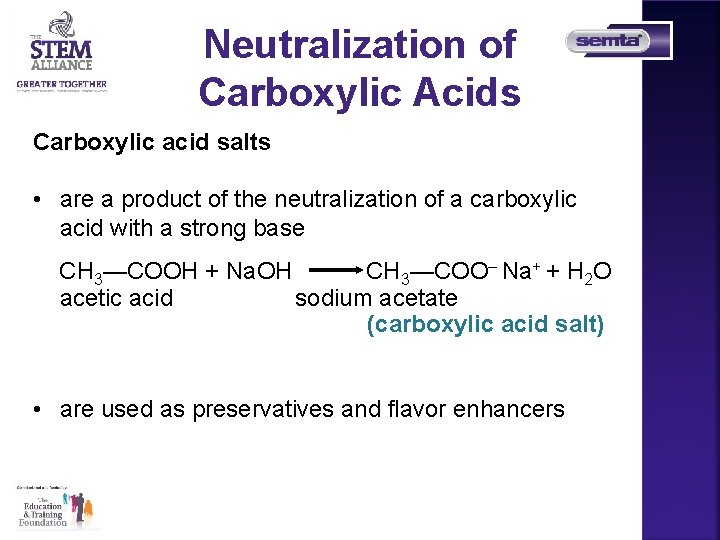
Neutralization of Carboxylic Acids Carboxylic acid salts • are a product of the neutralization of a carboxylic acid with a strong base CH 3—COOH + Na. OH CH 3—COO– Na+ + H 2 O acetic acid sodium acetate (carboxylic acid salt) • are used as preservatives and flavor enhancers

Carboxylic acid salts are used as preservatives and flavor enhancers such as • sodium propionate, which is used in cheese and breads • sodium benzoate, which inhibits growth of mold and bacteria and is added to fruit juices, margarine, relishes, salads, and jams • monosodium glutamate, MSG, which is added to meats, fish, vegetables, and baked goods to enhance flavor

Carboxylic Acid Salts Carboxylic acid salts • are ionic compounds with strong attractive forces between ions • are solids at room temperature • have high melting points • are usually soluble in water
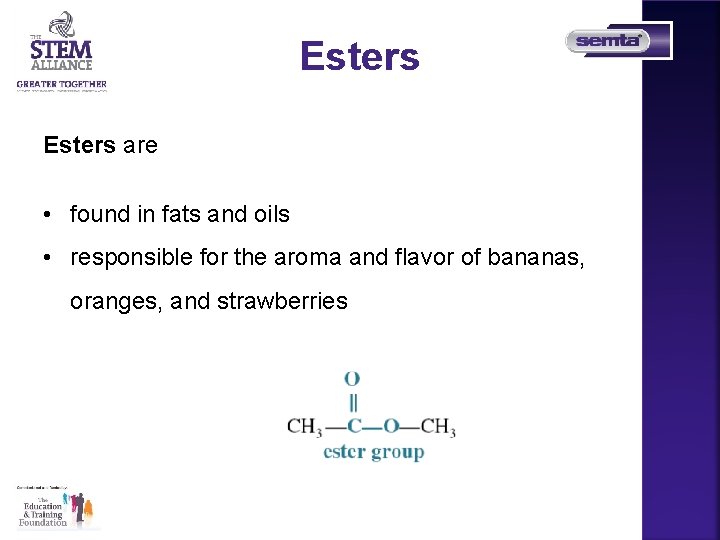
Esters are • found in fats and oils • responsible for the aroma and flavor of bananas, oranges, and strawberries
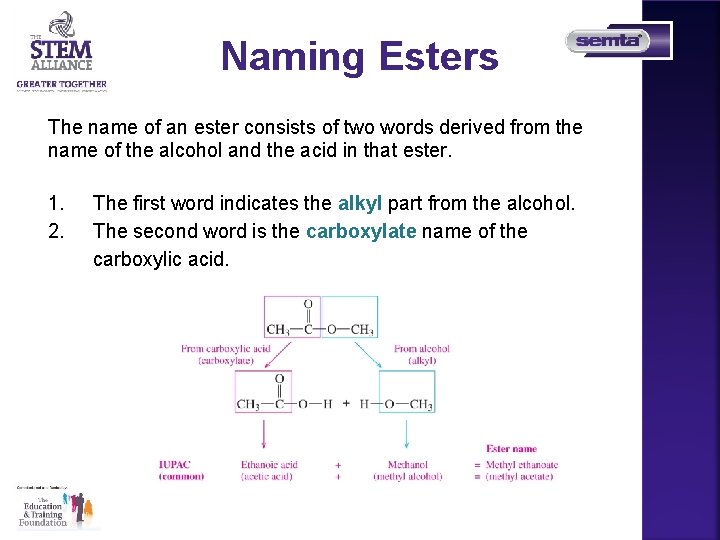
Naming Esters The name of an ester consists of two words derived from the name of the alcohol and the acid in that ester. 1. 2. The first word indicates the alkyl part from the alcohol. The second word is the carboxylate name of the carboxylic acid.
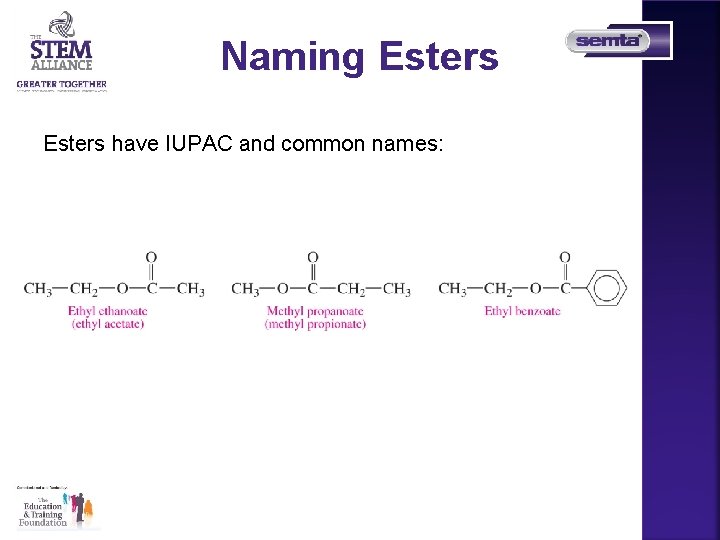
Naming Esters have IUPAC and common names:
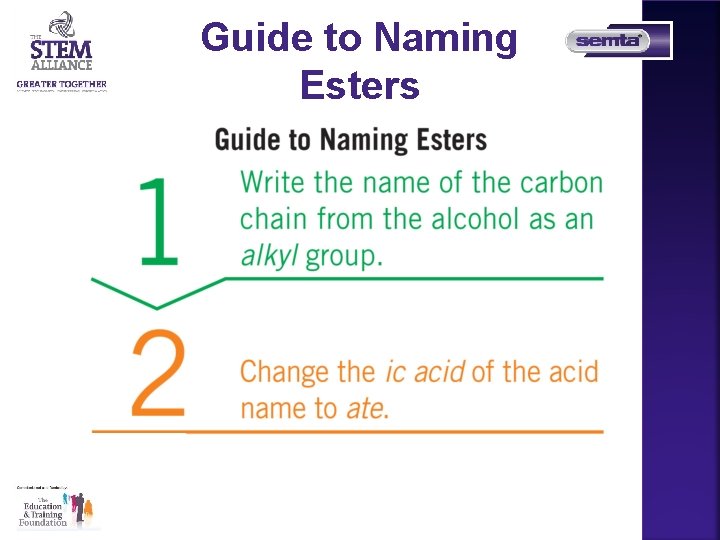
Guide to Naming Esters

Guide to Naming Esters What is the IUPAC name of the following ester? Step 1 Write the name of the carbon chain from the alcohol as an alkyl group.
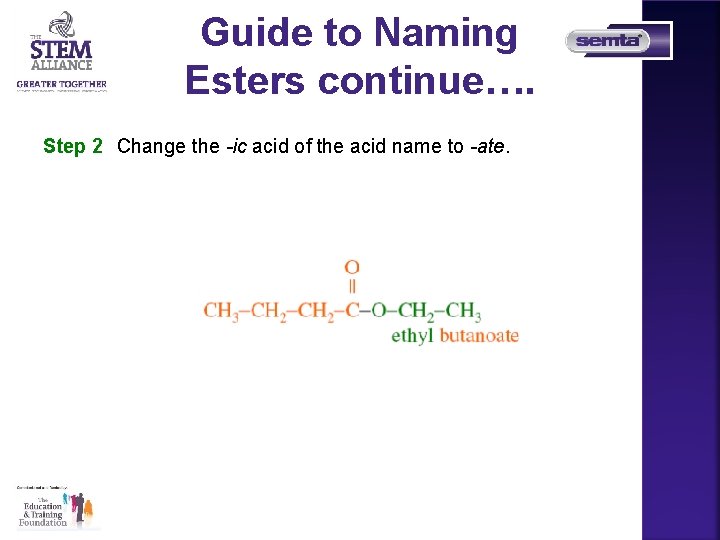
Guide to Naming Esters continue…. Step 2 Change the -ic acid of the acid name to -ate.
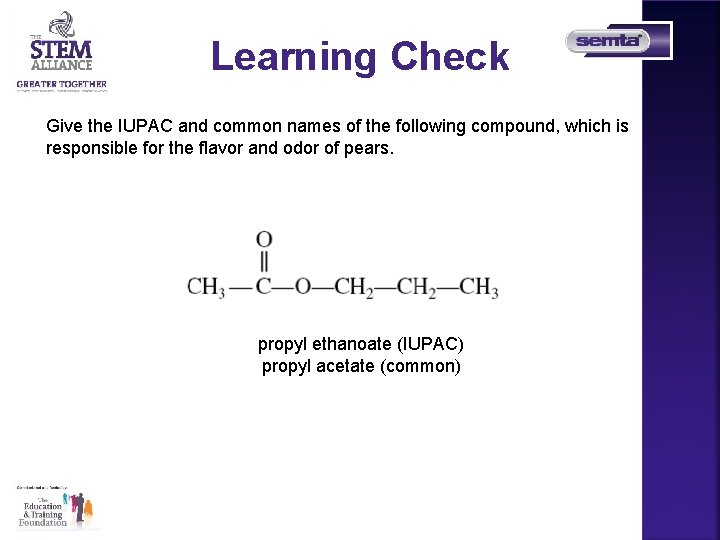
Learning Check Give the IUPAC and common names of the following compound, which is responsible for the flavor and odor of pears. propyl ethanoate (IUPAC) propyl acetate (common)
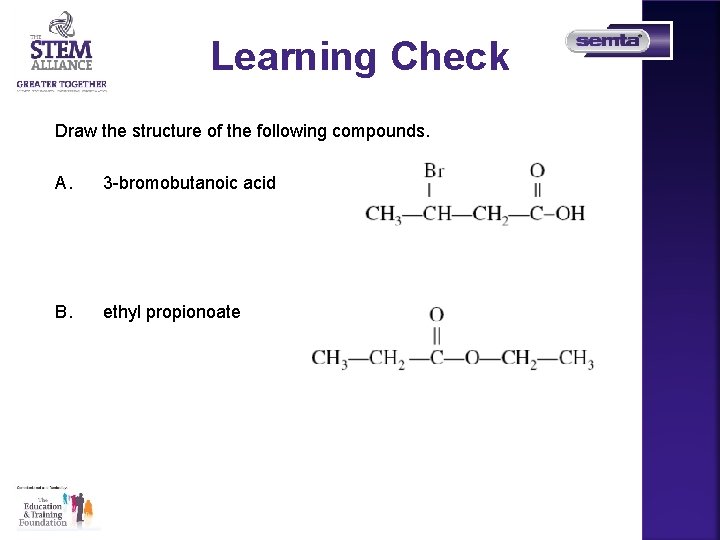
Learning Check Draw the structure of the following compounds. A. 3 -bromobutanoic acid B. ethyl propionoate
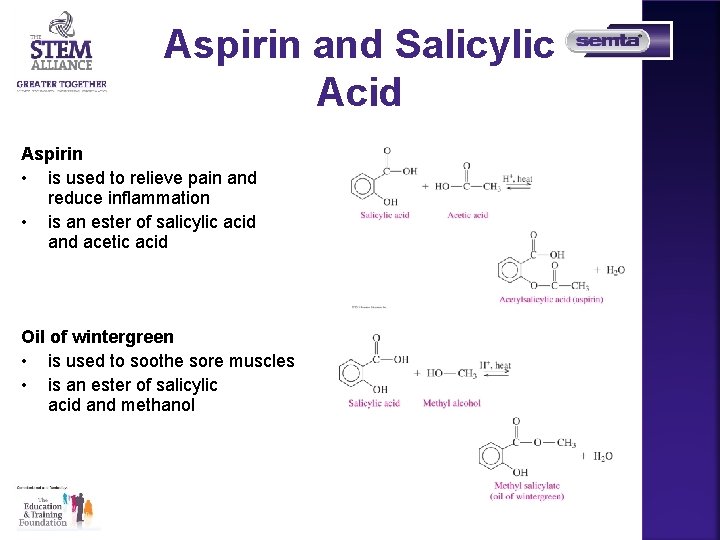
Aspirin and Salicylic Acid Aspirin • is used to relieve pain and reduce inflammation • is an ester of salicylic acid and acetic acid Oil of wintergreen • is used to soothe sore muscles • is an ester of salicylic acid and methanol
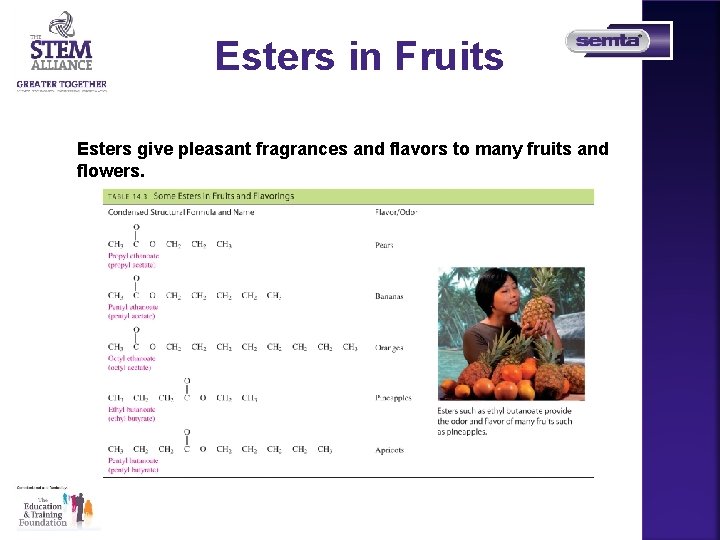
Esters in Fruits Esters give pleasant fragrances and flavors to many fruits and flowers.
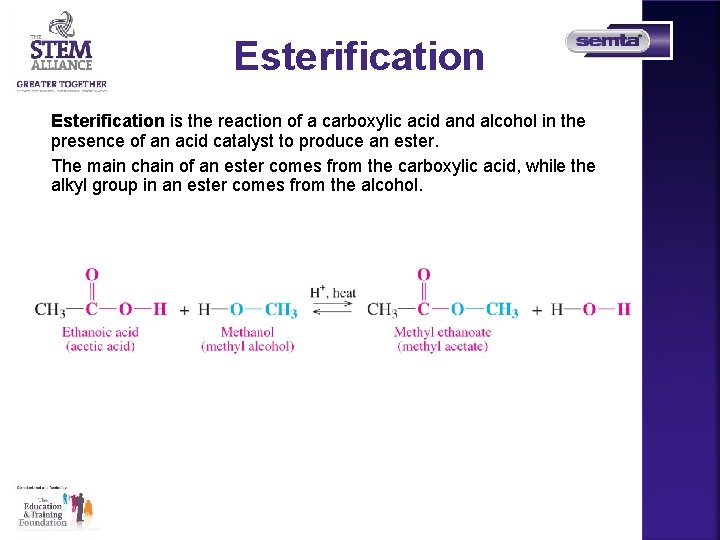
Esterification is the reaction of a carboxylic acid and alcohol in the presence of an acid catalyst to produce an ester. The main chain of an ester comes from the carboxylic acid, while the alkyl group in an ester comes from the alcohol.
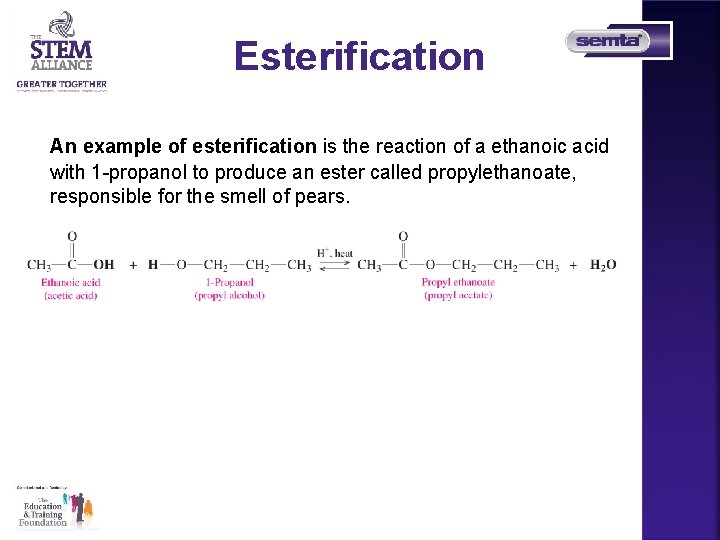
Esterification An example of esterification is the reaction of a ethanoic acid with 1 -propanol to produce an ester called propylethanoate, responsible for the smell of pears.
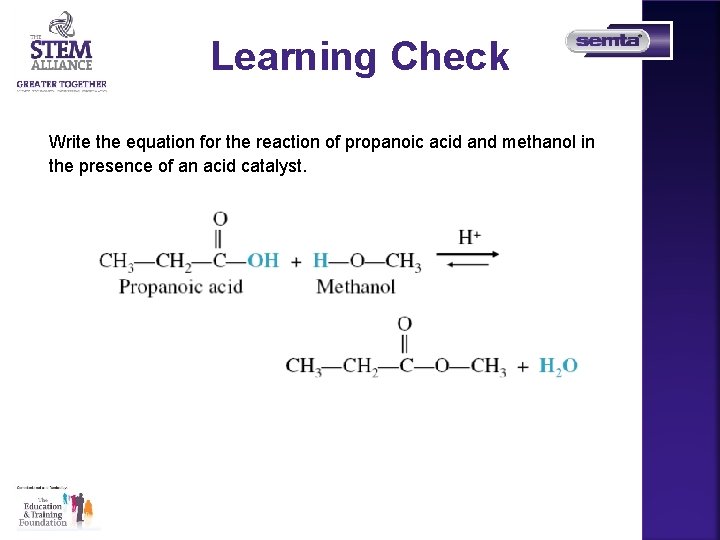
Learning Check Write the equation for the reaction of propanoic acid and methanol in the presence of an acid catalyst.
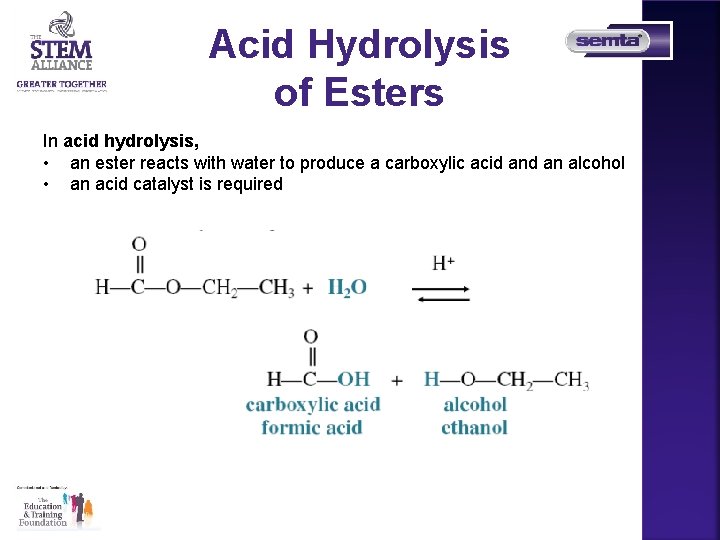
Acid Hydrolysis of Esters In acid hydrolysis, • an ester reacts with water to produce a carboxylic acid an alcohol • an acid catalyst is required
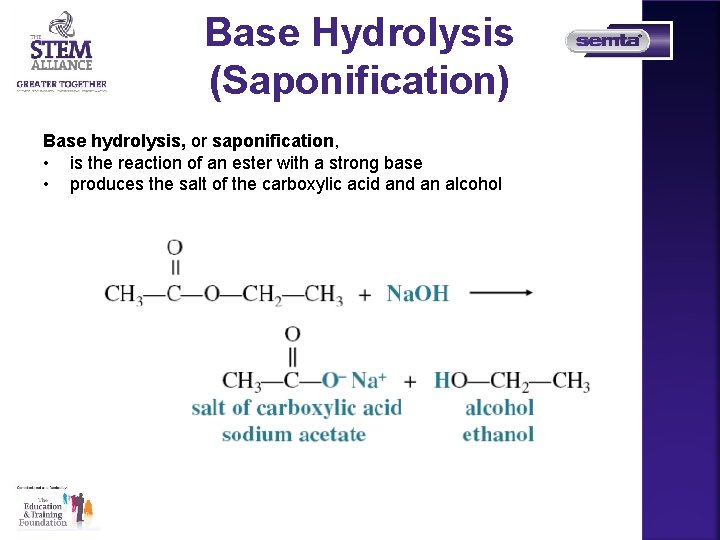
Base Hydrolysis (Saponification) Base hydrolysis, or saponification, • is the reaction of an ester with a strong base • produces the salt of the carboxylic acid an alcohol

Base Hydrolysis of Fatty Acids Produces Soaps

Cleaning Action of Soap A soap • contains a nonpolar end that dissolves in nonpolar fats and oils, and a polar end that dissolves in water • forms groups of soap molecules called micelles that dissolve in water and are washed away
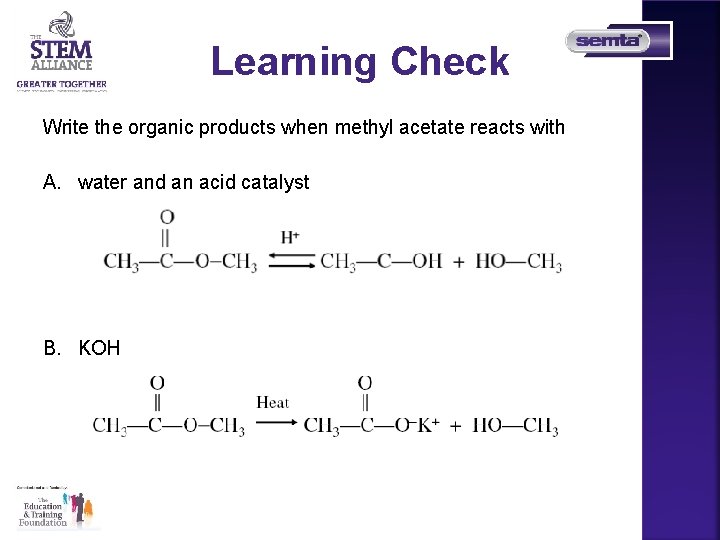
Learning Check Write the organic products when methyl acetate reacts with A. water and an acid catalyst B. KOH

For further information please contact The STEM Alliance enquiries@STEMalliance. uk or visit www. STEMalliance. uk
- Slides: 38