CARBOXYLIC ACIDS CONTENTS Structure of carboxylic acids Nomenclature

CARBOXYLIC ACIDS CONTENTS • Structure of carboxylic acids • Nomenclature • Physical properties of carboxylic acids • Preparation of carboxylic acids • Chemical properties of carboxylic acids • Esters

CARBOXYLIC ACIDS Before you start it would be helpful to… • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature rules for simple organic compounds • Recall the chemical properties of alkanes and alkenes

STRUCTURE OF CARBOXYLIC ACIDS • contain the carboxyl functional group COOH • the bonds are in a planar arrangement
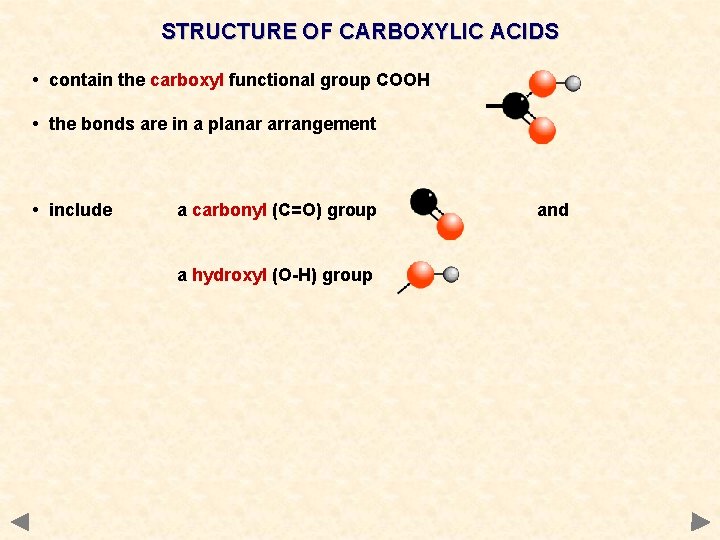
STRUCTURE OF CARBOXYLIC ACIDS • contain the carboxyl functional group COOH • the bonds are in a planar arrangement • include a carbonyl (C=O) group a hydroxyl (O-H) group and
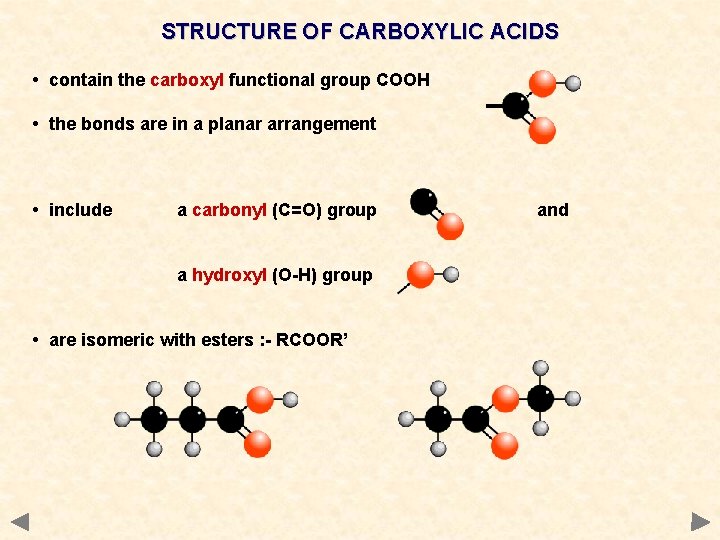
STRUCTURE OF CARBOXYLIC ACIDS • contain the carboxyl functional group COOH • the bonds are in a planar arrangement • include a carbonyl (C=O) group a hydroxyl (O-H) group • are isomeric with esters : - RCOOR’ and
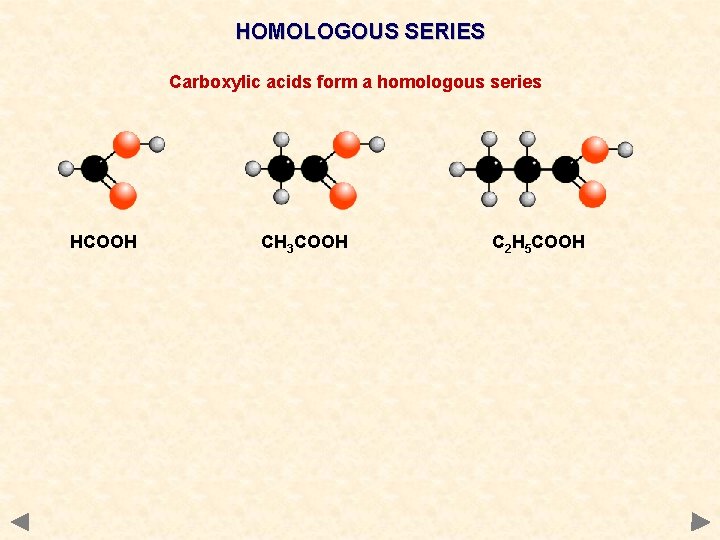
HOMOLOGOUS SERIES Carboxylic acids form a homologous series HCOOH CH 3 COOH C 2 H 5 COOH
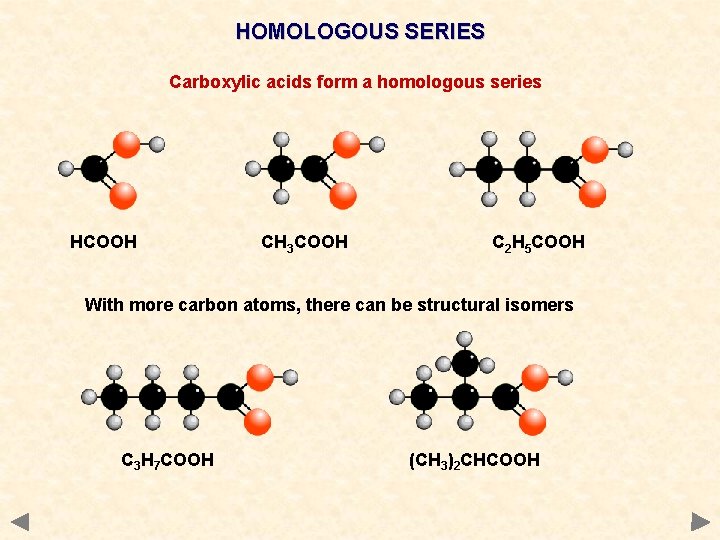
HOMOLOGOUS SERIES Carboxylic acids form a homologous series HCOOH CH 3 COOH C 2 H 5 COOH With more carbon atoms, there can be structural isomers C 3 H 7 COOH (CH 3)2 CHCOOH
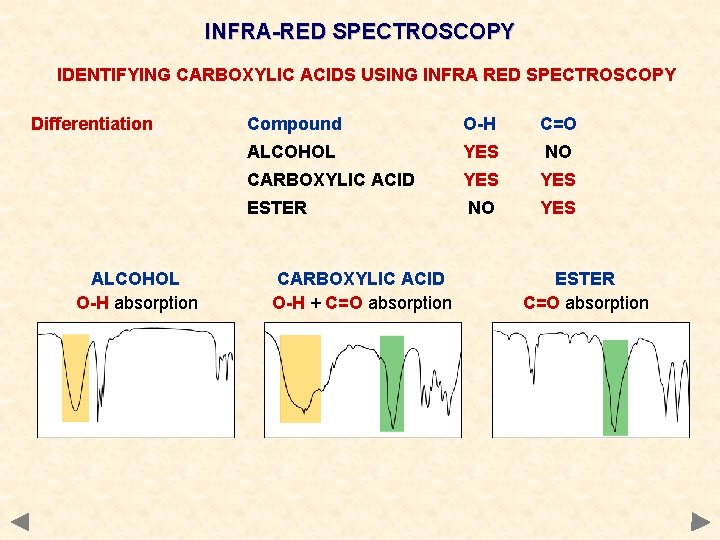
INFRA-RED SPECTROSCOPY IDENTIFYING CARBOXYLIC ACIDS USING INFRA RED SPECTROSCOPY Differentiation ALCOHOL O-H absorption Compound O-H C=O ALCOHOL YES NO CARBOXYLIC ACID YES ESTER NO YES CARBOXYLIC ACID O-H + C=O absorption ESTER C=O absorption

NAMING CARBOXYLIC ACIDS Acids are named according to standard IUPAC rules • select the longest chain of C atoms containing the COOH group; • remove the e and add oic acid after the basic name • number the chain starting from the end nearer the COOH group • as in alkanes, prefix with alkyl substituents • side chain positions are based on the C in COOH being 1 e. g. CH 3 - CH(CH 3) - CH 2 - COOH is called 4 -methylpentanoic acid

NAMING CARBOXYLIC ACIDS Acids are named according to standard IUPAC rules • select the longest chain of C atoms containing the COOH group; • remove the e and add oic acid after the basic name • number the chain starting from the end nearer the COOH group • as in alkanes, prefix with alkyl substituents • side chain positions are based on the C in COOH being 1 METHANOIC ACID PROPANOIC ACID
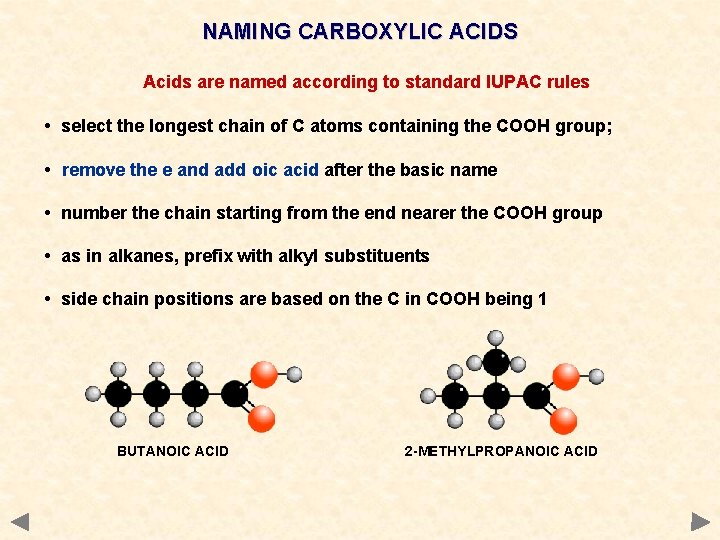
NAMING CARBOXYLIC ACIDS Acids are named according to standard IUPAC rules • select the longest chain of C atoms containing the COOH group; • remove the e and add oic acid after the basic name • number the chain starting from the end nearer the COOH group • as in alkanes, prefix with alkyl substituents • side chain positions are based on the C in COOH being 1 BUTANOIC ACID 2 -METHYLPROPANOIC ACID

NAMING CARBOXYLIC ACIDS Acids are named according to standard IUPAC rules Many carboxylic acids are still known under their trivial names, some having been called after characteristic properties or their origin. Formula HCOOH CH 3 COOH C 6 H 5 COOH Systematic name methanoic acid benzenecarboxylic acid (trivial name) formic acid acetic acid benzoic acid origin of name latin for ant latin for vinegar from benzene
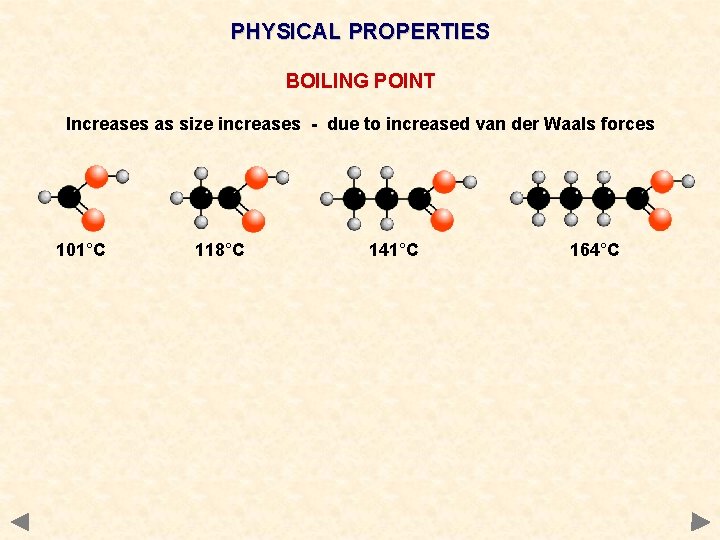
PHYSICAL PROPERTIES BOILING POINT Increases as size increases - due to increased van der Waals forces 101°C 118°C 141°C 164°C
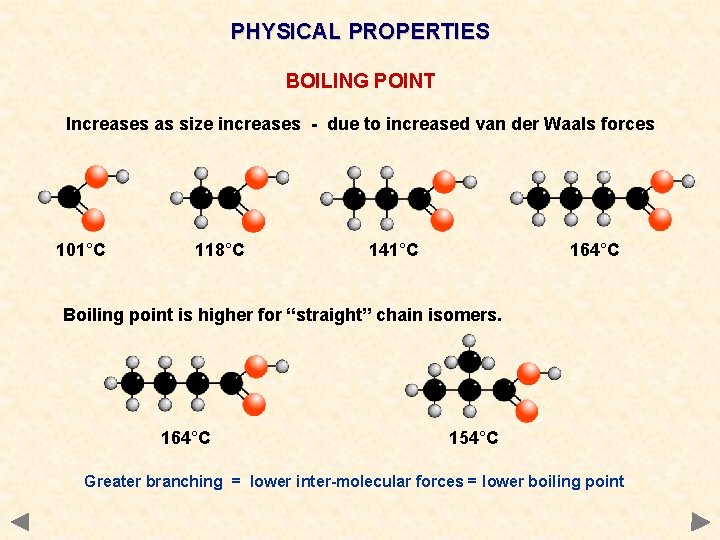
PHYSICAL PROPERTIES BOILING POINT Increases as size increases - due to increased van der Waals forces 101°C 118°C 141°C 164°C Boiling point is higher for “straight” chain isomers. 164°C 154°C Greater branching = lower inter-molecular forces = lower boiling point
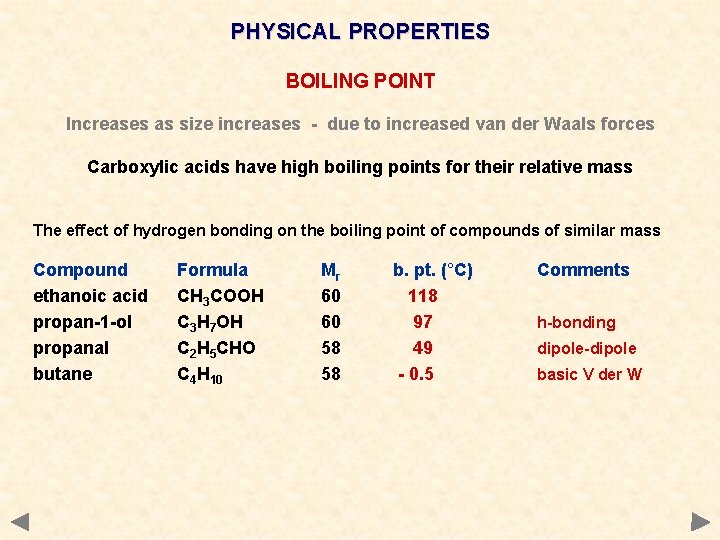
PHYSICAL PROPERTIES BOILING POINT Increases as size increases - due to increased van der Waals forces Carboxylic acids have high boiling points for their relative mass The effect of hydrogen bonding on the boiling point of compounds of similar mass Compound ethanoic acid propan-1 -ol propanal butane Formula CH 3 COOH C 3 H 7 OH C 2 H 5 CHO C 4 H 10 Mr 60 60 58 58 b. pt. (°C) 118 97 49 - 0. 5 Comments h-bonding dipole-dipole basic V der W
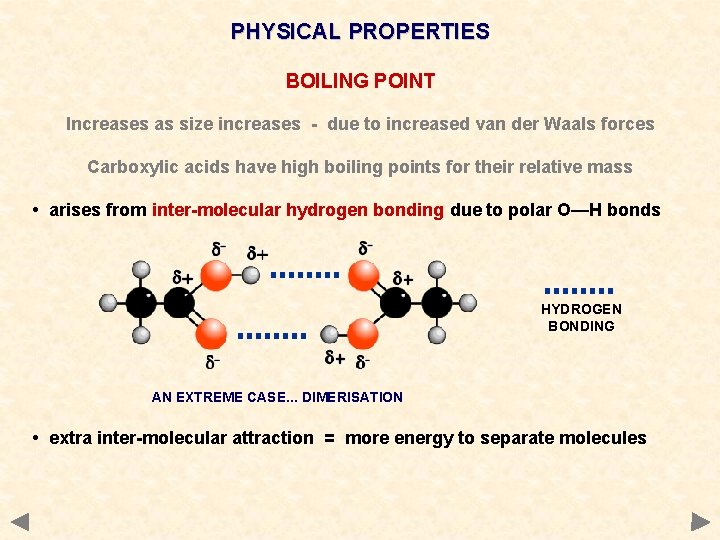
PHYSICAL PROPERTIES BOILING POINT Increases as size increases - due to increased van der Waals forces Carboxylic acids have high boiling points for their relative mass • arises from inter-molecular hydrogen bonding due to polar O—H bonds HYDROGEN BONDING AN EXTREME CASE. . . DIMERISATION • extra inter-molecular attraction = more energy to separate molecules
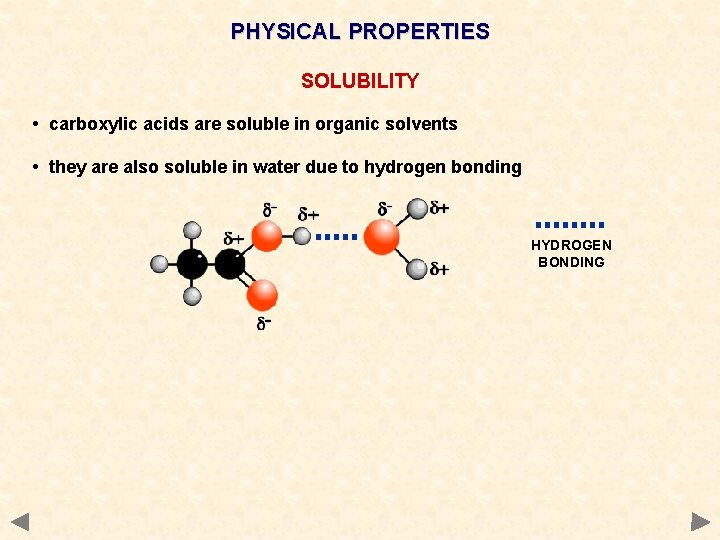
PHYSICAL PROPERTIES SOLUBILITY • carboxylic acids are soluble in organic solvents • they are also soluble in water due to hydrogen bonding HYDROGEN BONDING
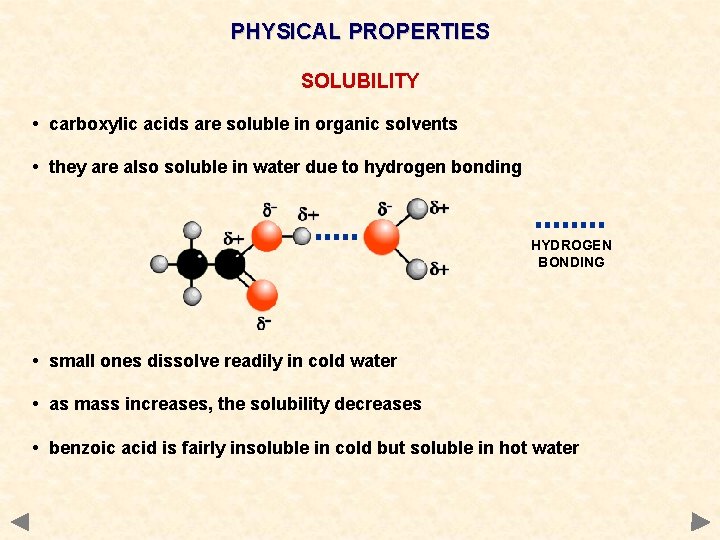
PHYSICAL PROPERTIES SOLUBILITY • carboxylic acids are soluble in organic solvents • they are also soluble in water due to hydrogen bonding HYDROGEN BONDING • small ones dissolve readily in cold water • as mass increases, the solubility decreases • benzoic acid is fairly insoluble in cold but soluble in hot water

CHEMICAL PROPERTIES ACIDITY weak acids RCOOH + H 2 O(l) RCOO¯(aq) + H 3 O+(aq) form salts RCOOH + Na. OH(aq) ——> RCOO¯Na+(aq) + H 2 O(l)

CHEMICAL PROPERTIES ACIDITY weak acids RCOOH + H 2 O(l) RCOO¯(aq) + H 3 O+(aq) form salts RCOOH + Na. OH(aq) ——> RCOO¯Na+(aq) + H 2 O(l) QUALITATIVE ANALYSIS Carboxylic acids are strong enough acids to liberate CO 2 from carbonates Phenols are also acidic but not are not strong enough to liberate CO 2

ESTERIFICATION Reagent(s) alcohol + strong acid catalyst (e. g. conc. H 2 SO 4 ) Conditions reflux Product ester Equation e. g. CH 3 CH 2 OH(l) + CH 3 COOH(l) ethanol ethanoic acid CH 3 COOC 2 H 5(l) + H 2 O(l) ethyl ethanoate

ESTERIFICATION Reagent(s) alcohol + strong acid catalyst (e. g. conc. H 2 SO 4 ) Conditions reflux Product ester Equation Notes e. g. CH 3 CH 2 OH(l) + CH 3 COOH(l) ethanol ethanoic acid CH 3 COOC 2 H 5(l) + H 2 O(l) ethyl ethanoate Conc. H 2 SO 4 is a dehydrating agent - it removes water causing the equilibrium to move to the right and thus increases the yield of the ester

ESTERIFICATION Reagent(s) alcohol + strong acid catalyst (e. g conc. H 2 SO 4 ) Conditions reflux Product ester Equation e. g. CH 3 CH 2 OH(l) + CH 3 COOH(l) ethanol ethanoic acid CH 3 COOC 2 H 5(l) + H 2 O(l) ethyl ethanoate Notes Conc. H 2 SO 4 is a dehydrating agent - it removes water causing the equilibrium to move to the right and thus increases the yield of the ester Naming esters Named from the original alcohol and carboxylic acid CH 3 OH + CH 3 COOH from ethanoic acid CH 3 COOCH 3 + H 2 O CH 3 COOCH 3 METHYL ETHANOATE from methanol
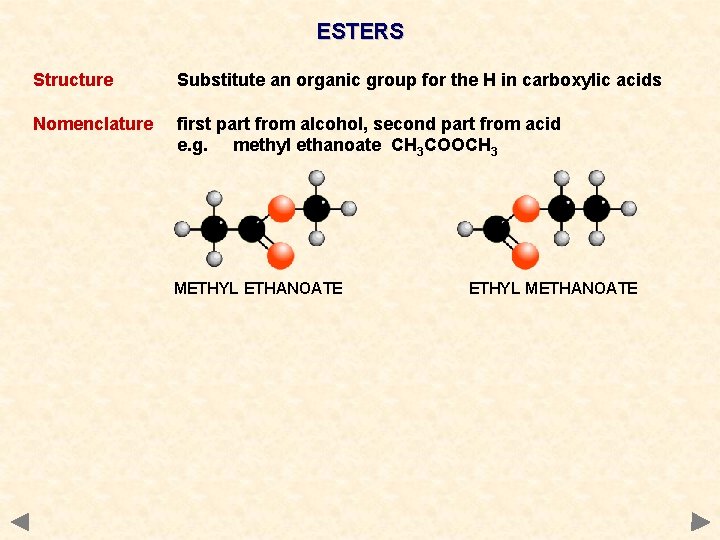
ESTERS Structure Substitute an organic group for the H in carboxylic acids Nomenclature first part from alcohol, second part from acid e. g. methyl ethanoate CH 3 COOCH 3 METHYL ETHANOATE ETHYL METHANOATE
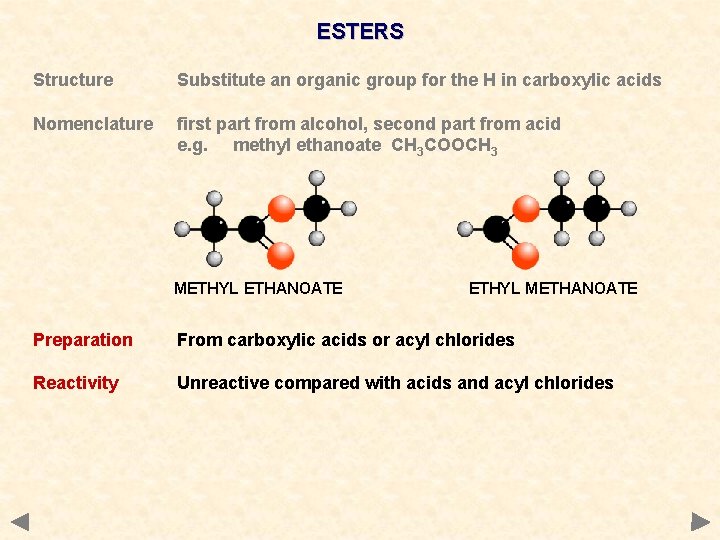
ESTERS Structure Substitute an organic group for the H in carboxylic acids Nomenclature first part from alcohol, second part from acid e. g. methyl ethanoate CH 3 COOCH 3 METHYL ETHANOATE ETHYL METHANOATE Preparation From carboxylic acids or acyl chlorides Reactivity Unreactive compared with acids and acyl chlorides
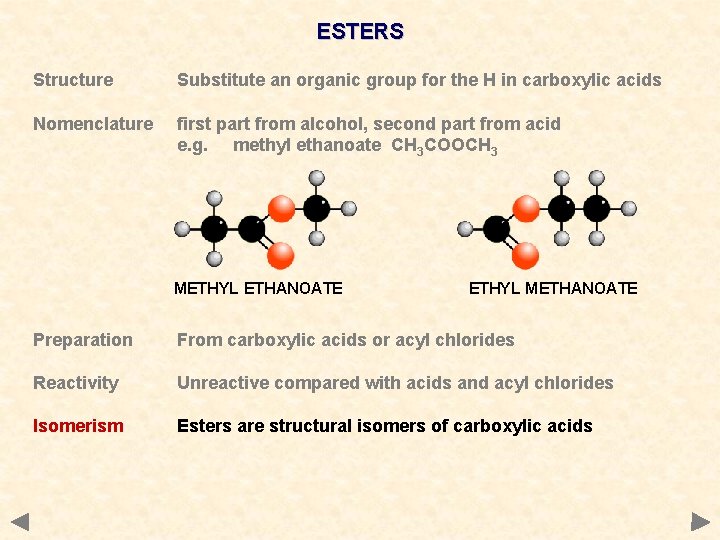
ESTERS Structure Substitute an organic group for the H in carboxylic acids Nomenclature first part from alcohol, second part from acid e. g. methyl ethanoate CH 3 COOCH 3 METHYL ETHANOATE ETHYL METHANOATE Preparation From carboxylic acids or acyl chlorides Reactivity Unreactive compared with acids and acyl chlorides Isomerism Esters are structural isomers of carboxylic acids
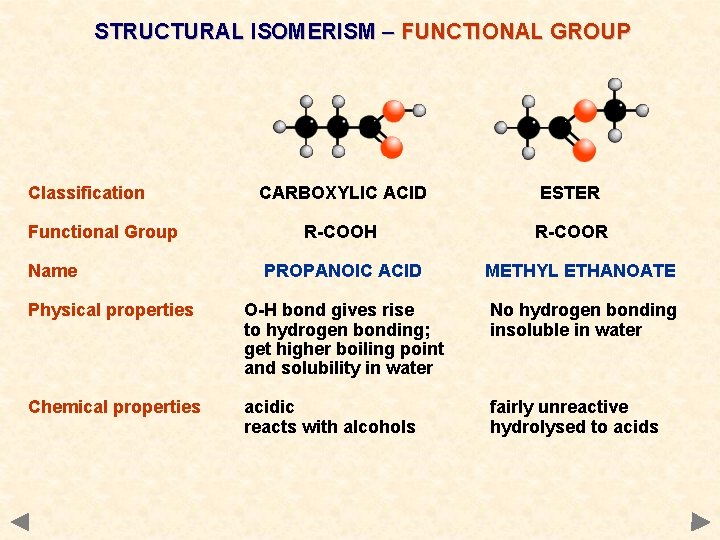
STRUCTURAL ISOMERISM – FUNCTIONAL GROUP Classification Functional Group Name CARBOXYLIC ACID ESTER R-COOH R-COOR PROPANOIC ACID METHYL ETHANOATE Physical properties O-H bond gives rise to hydrogen bonding; get higher boiling point and solubility in water No hydrogen bonding insoluble in water Chemical properties acidic reacts with alcohols fairly unreactive hydrolysed to acids
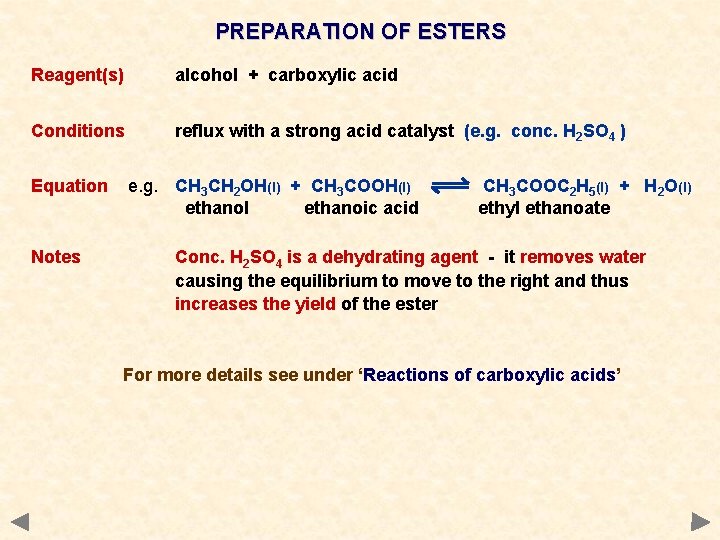
PREPARATION OF ESTERS Reagent(s) alcohol + carboxylic acid Conditions reflux with a strong acid catalyst (e. g. conc. H 2 SO 4 ) Equation Notes e. g. CH 3 CH 2 OH(l) + CH 3 COOH(l) ethanol ethanoic acid CH 3 COOC 2 H 5(l) + H 2 O(l) ethyl ethanoate Conc. H 2 SO 4 is a dehydrating agent - it removes water causing the equilibrium to move to the right and thus increases the yield of the ester For more details see under ‘Reactions of carboxylic acids’

HYDROLYSIS OF ESTERS Hydrolysis is the opposite of esterification ESTER + WATER CARBOXYLIC ACID + ALCOHOL HCOOH METHANOIC ACID ETHYL METHANOATE + C 2 H 5 OH ETHANOL
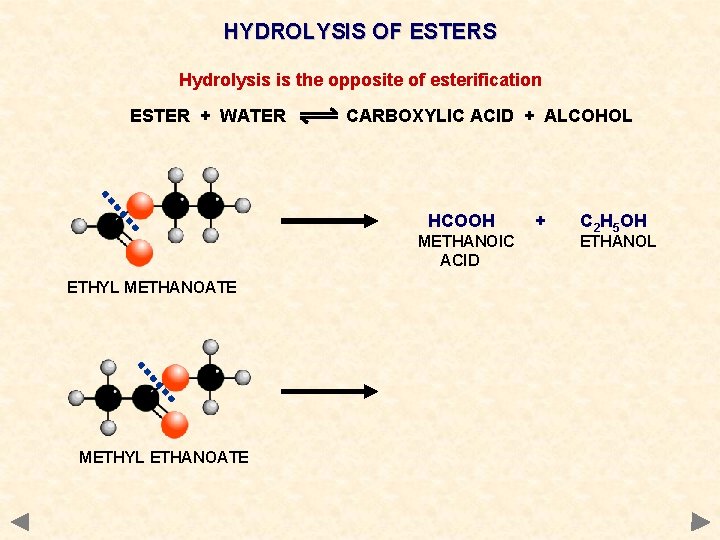
HYDROLYSIS OF ESTERS Hydrolysis is the opposite of esterification ESTER + WATER CARBOXYLIC ACID + ALCOHOL HCOOH METHANOIC ACID ETHYL METHANOATE METHYL ETHANOATE + C 2 H 5 OH ETHANOL
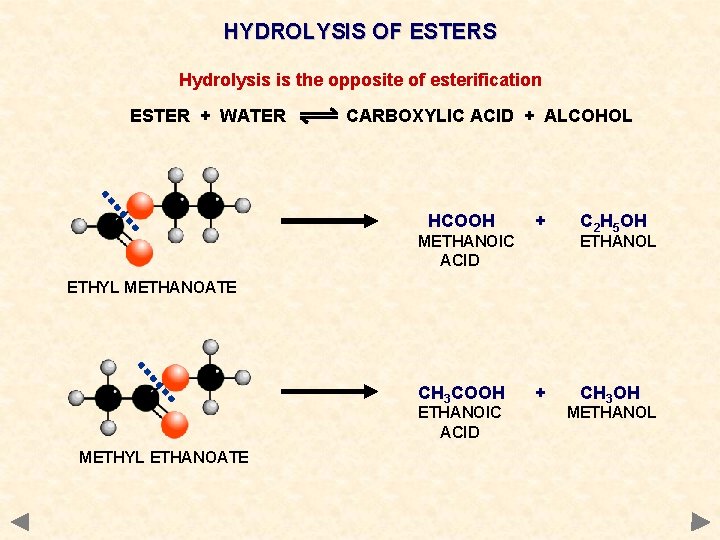
HYDROLYSIS OF ESTERS Hydrolysis is the opposite of esterification ESTER + WATER CARBOXYLIC ACID + ALCOHOL HCOOH + METHANOIC ACID C 2 H 5 OH ETHANOL ETHYL METHANOATE CH 3 COOH ETHANOIC ACID METHYL ETHANOATE + CH 3 OH METHANOL
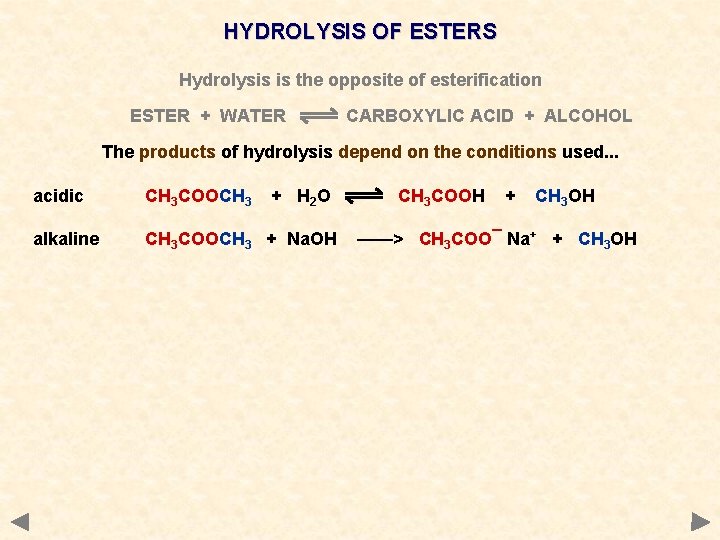
HYDROLYSIS OF ESTERS Hydrolysis is the opposite of esterification ESTER + WATER CARBOXYLIC ACID + ALCOHOL The products of hydrolysis depend on the conditions used. . . acidic CH 3 COOCH 3 + H 2 O alkaline CH 3 COOCH 3 + Na. OH CH 3 COOH + CH 3 OH ——> CH 3 COO¯ Na+ + CH 3 OH

HYDROLYSIS OF ESTERS Hydrolysis is the opposite of esterification ESTER + WATER CARBOXYLIC ACID + ALCOHOL The products of hydrolysis depend on the conditions used. . . acidic CH 3 COOCH 3 + H 2 O alkaline CH 3 COOCH 3 + Na. OH CH 3 COOH + CH 3 OH ——> CH 3 COO¯ Na+ + CH 3 OH If the hydrolysis takes place under alkaline conditions, the organic product is a water soluble ionic salt
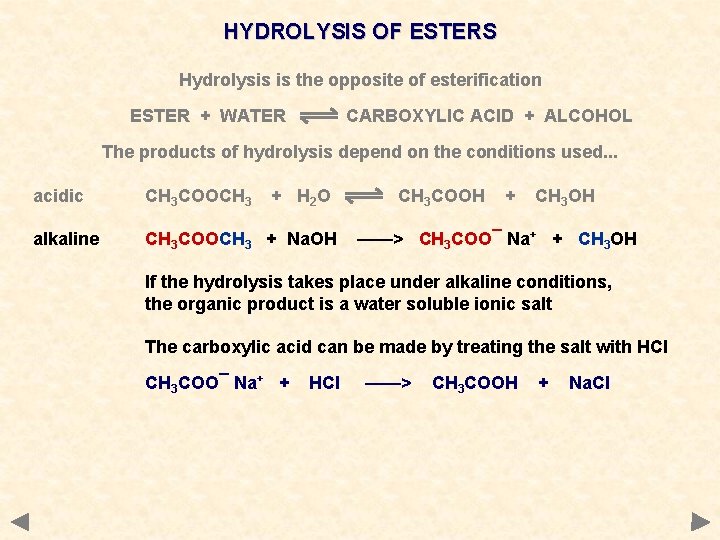
HYDROLYSIS OF ESTERS Hydrolysis is the opposite of esterification ESTER + WATER CARBOXYLIC ACID + ALCOHOL The products of hydrolysis depend on the conditions used. . . acidic CH 3 COOCH 3 + H 2 O alkaline CH 3 COOCH 3 + Na. OH CH 3 COOH + CH 3 OH ——> CH 3 COO¯ Na+ + CH 3 OH If the hydrolysis takes place under alkaline conditions, the organic product is a water soluble ionic salt The carboxylic acid can be made by treating the salt with HCl CH 3 COO¯ Na+ + HCl ——> CH 3 COOH + Na. Cl
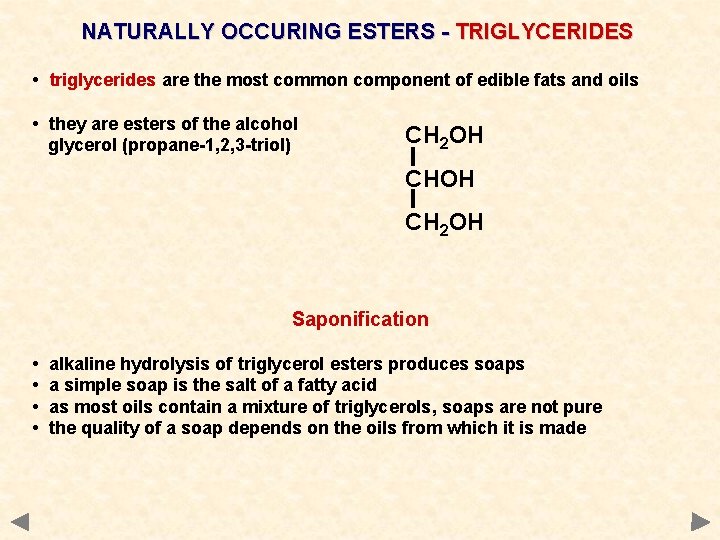
NATURALLY OCCURING ESTERS - TRIGLYCERIDES • triglycerides are the most common component of edible fats and oils • they are esters of the alcohol glycerol (propane-1, 2, 3 -triol) CH 2 OH Saponification • • alkaline hydrolysis of triglycerol esters produces soaps a simple soap is the salt of a fatty acid as most oils contain a mixture of triglycerols, soaps are not pure the quality of a soap depends on the oils from which it is made

USES OF ESTERS Despite being fairly chemically unreactive, esters are useful as. . . • flavourings apple pear banana pineapple rum • solvents nail varnish remover - ethyl ethanoate • plasticisers 2 -methylbutanoate 3 -methylbutylethanoate 1 -methylbutylethanoate butylbutanoate 2 -methylpropanoate
- Slides: 36