Carboxylic Acids Carboxylic Acids Organic compounds with carboxyl

Carboxylic Acids

Carboxylic Acids • Organic compounds with carboxyl groups
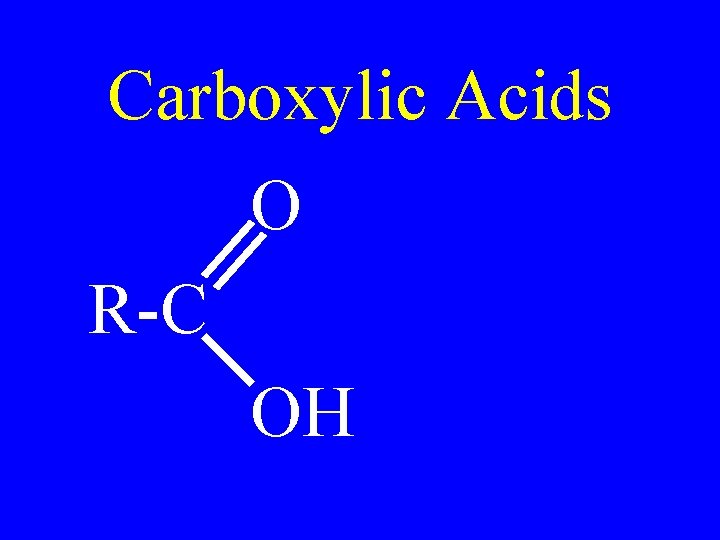
Carboxylic Acids O R-C OH

Common Acids
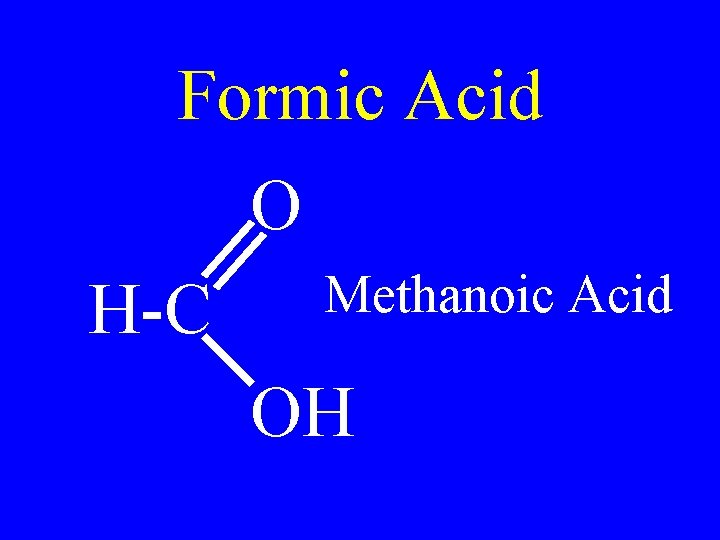
Formic Acid O H-C Methanoic Acid OH
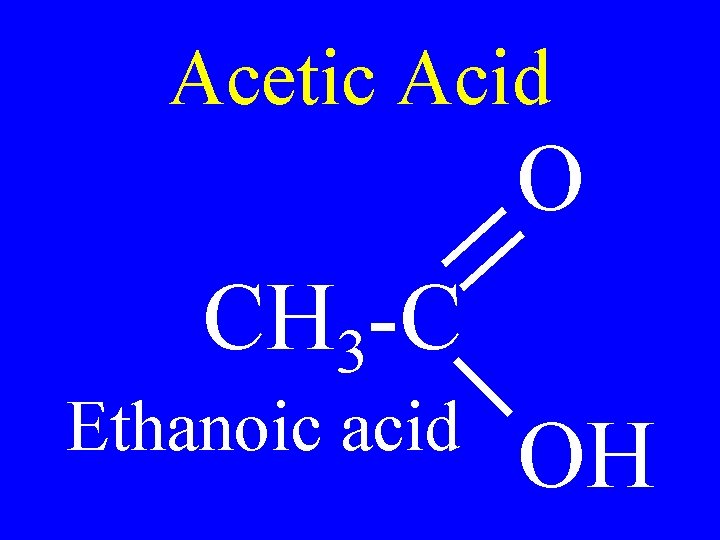
Acetic Acid O CH 3 -C Ethanoic acid OH
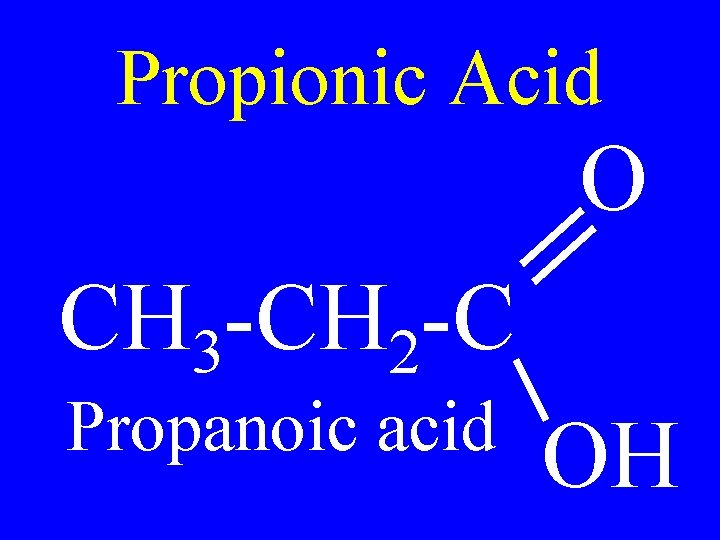
Propionic Acid O CH 3 -CH 2 -C Propanoic acid OH
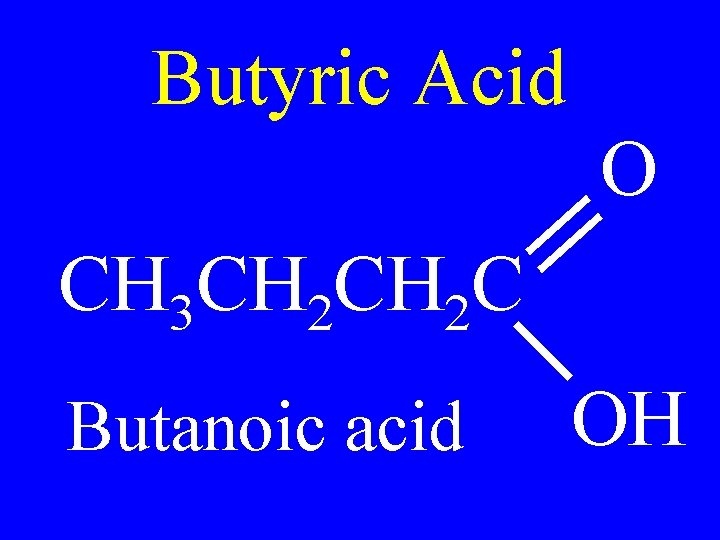
Butyric Acid O CH 3 CH 2 C Butanoic acid OH

Fatty Acids • Continuous-chain carboxylic acids isolated from fats
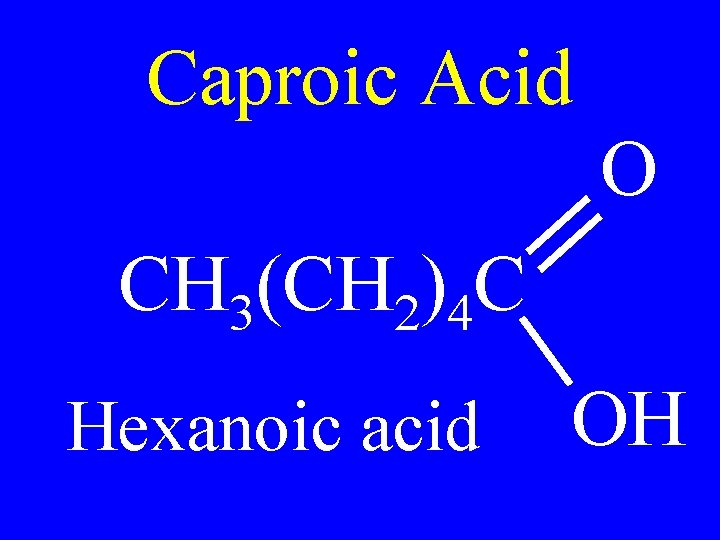
Caproic Acid O CH 3(CH 2)4 C Hexanoic acid OH
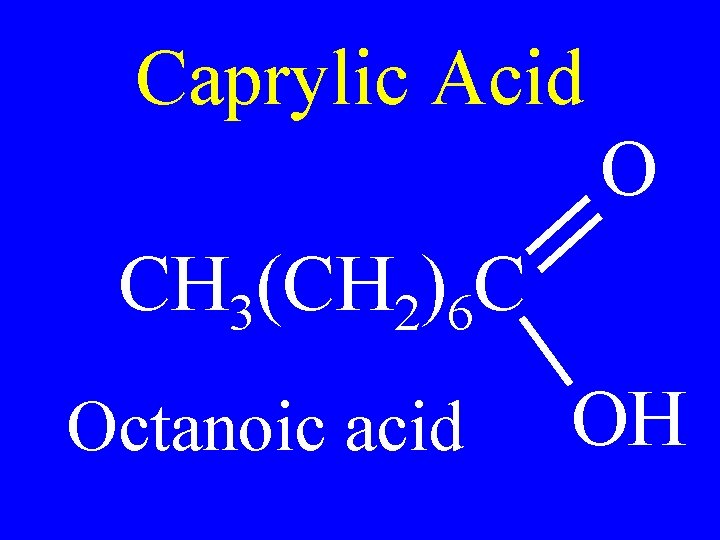
Caprylic Acid O CH 3(CH 2)6 C Octanoic acid OH
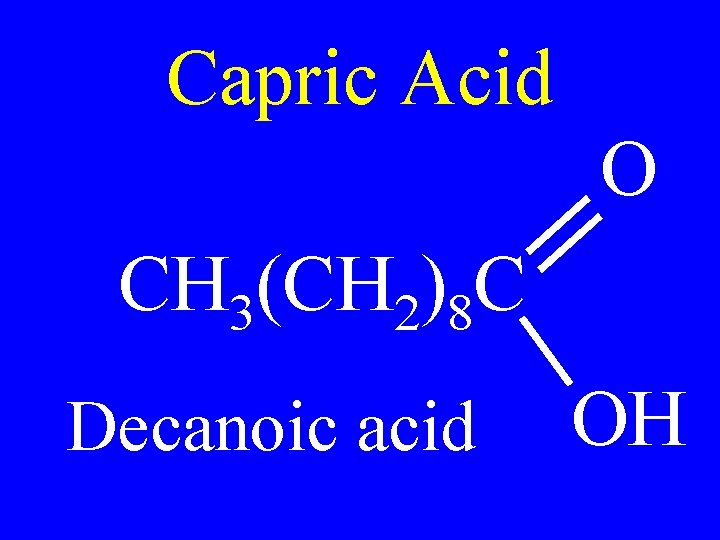
Capric Acid O CH 3(CH 2)8 C Decanoic acid OH
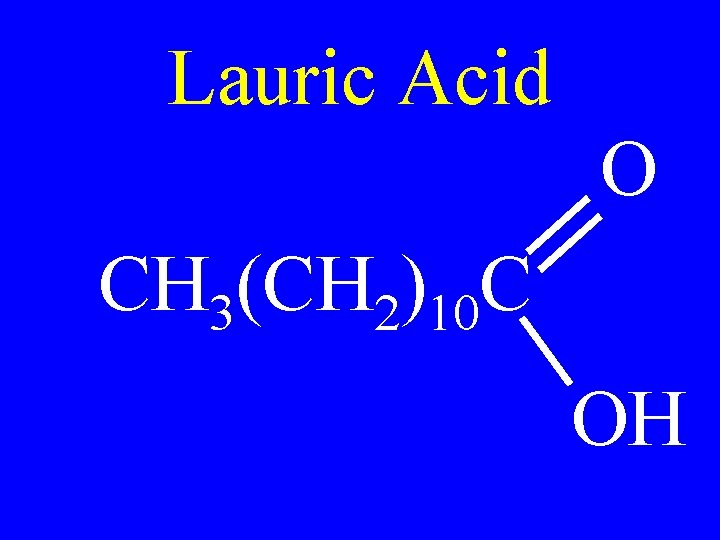
Lauric Acid O CH 3(CH 2)10 C OH
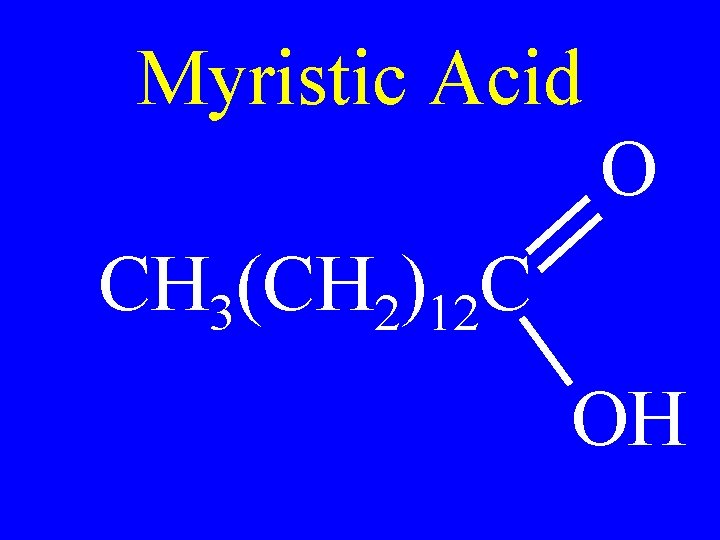
Myristic Acid O CH 3(CH 2)12 C OH
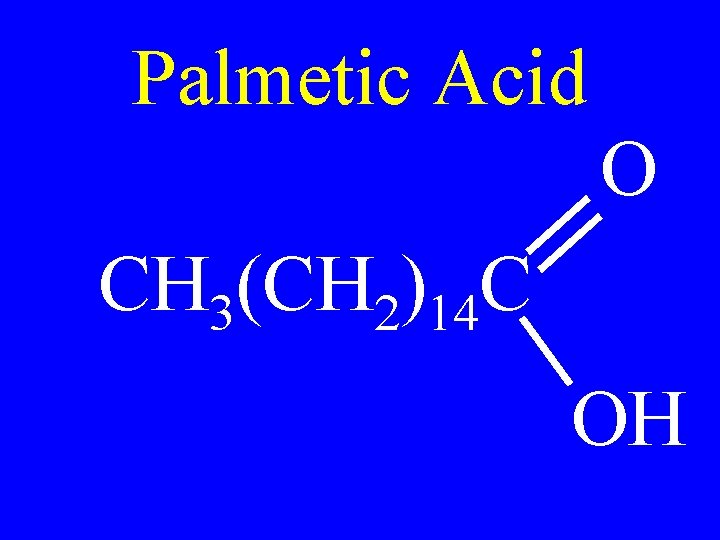
Palmetic Acid O CH 3(CH 2)14 C OH
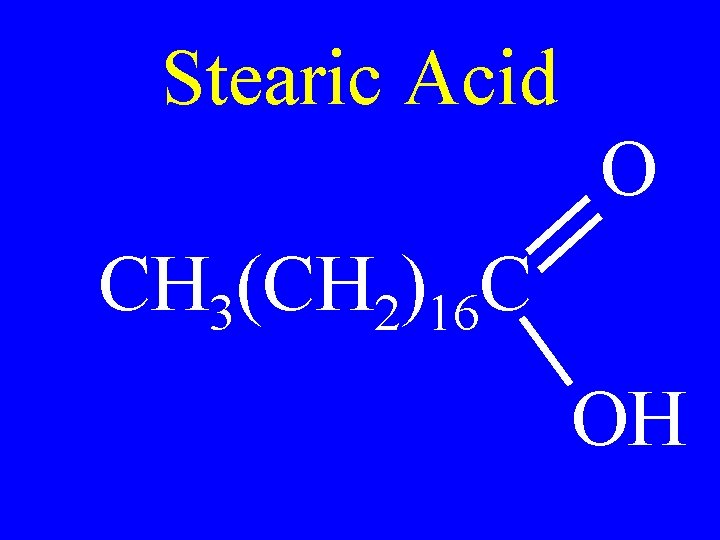
Stearic Acid O CH 3(CH 2)16 C OH

Unsaturated F A • Oleic acid • Linolenic Acid
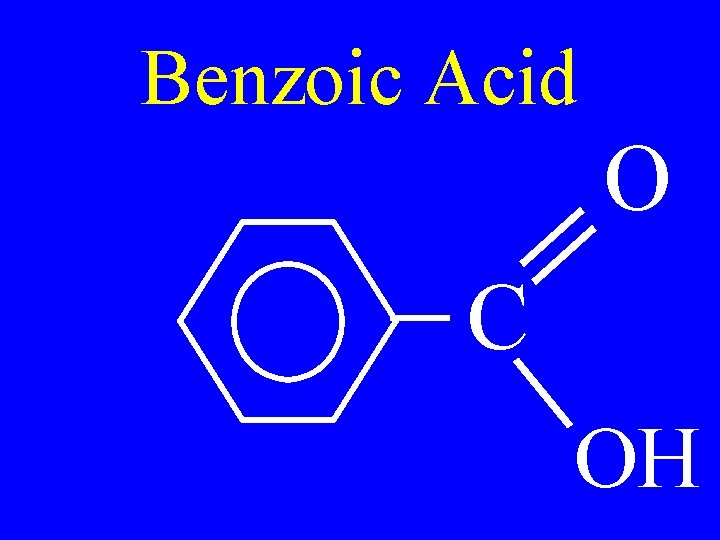
Benzoic Acid O C OH

Dicarboxylic Acids • Organic compounds with two carboxyl groups
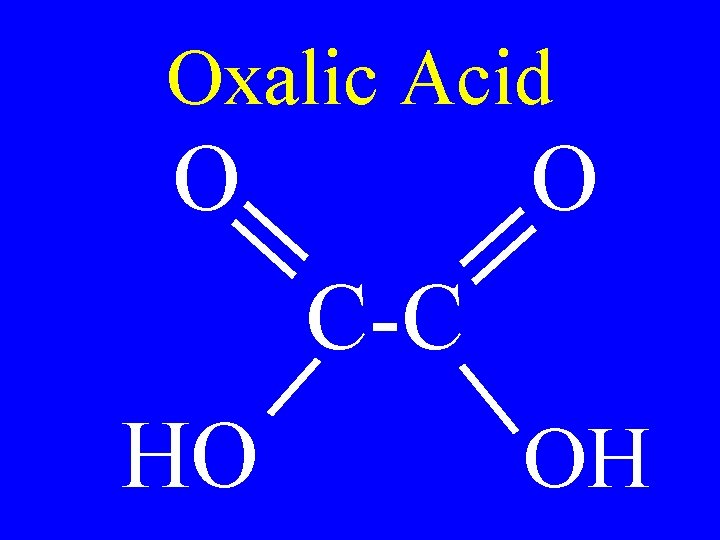
Oxalic Acid O O C-C HO OH
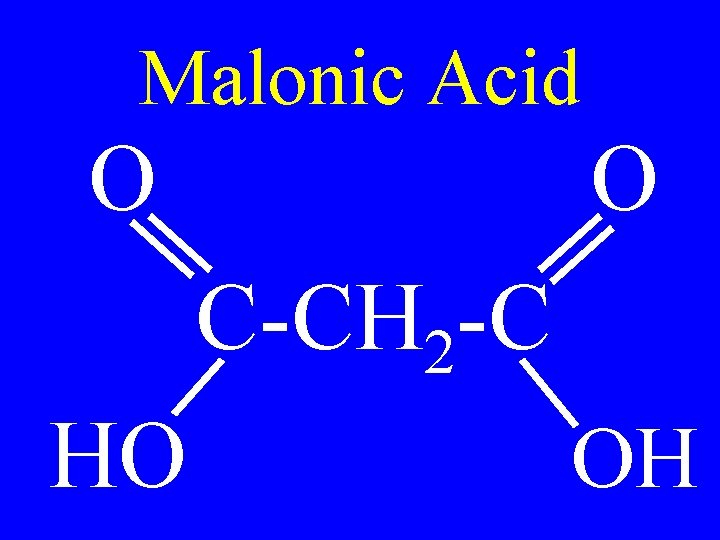
Malonic Acid O O C-CH 2 -C HO OH
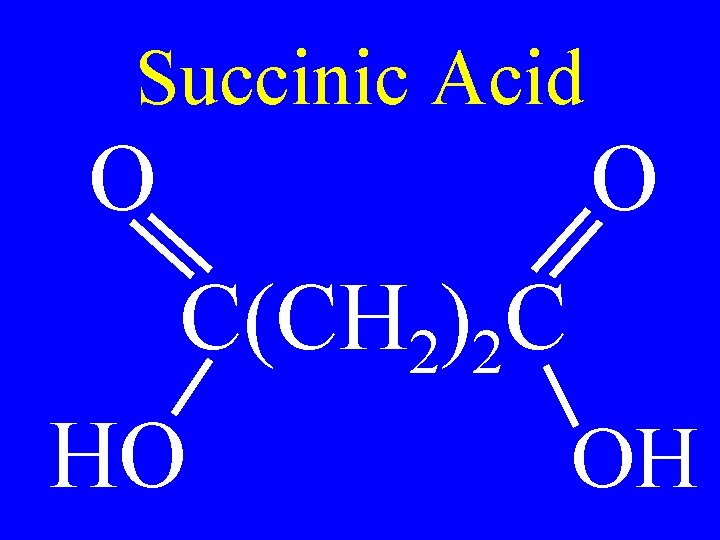
Succinic Acid O O C(CH 2)2 C HO OH
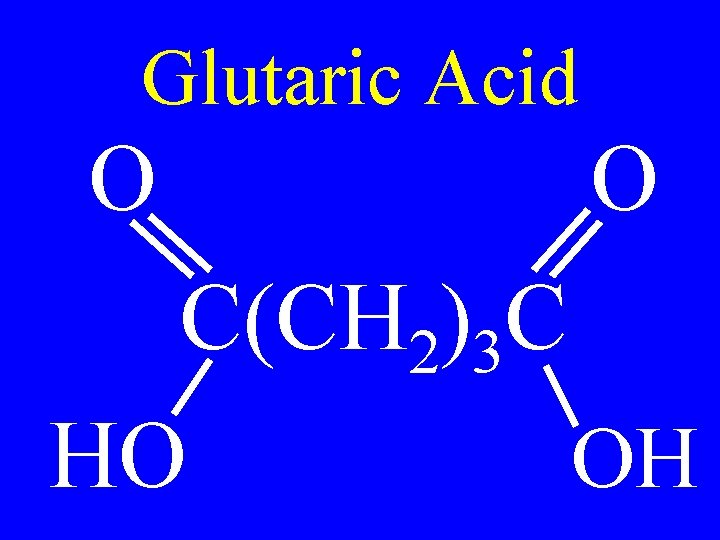
Glutaric Acid O O C(CH 2)3 C HO OH
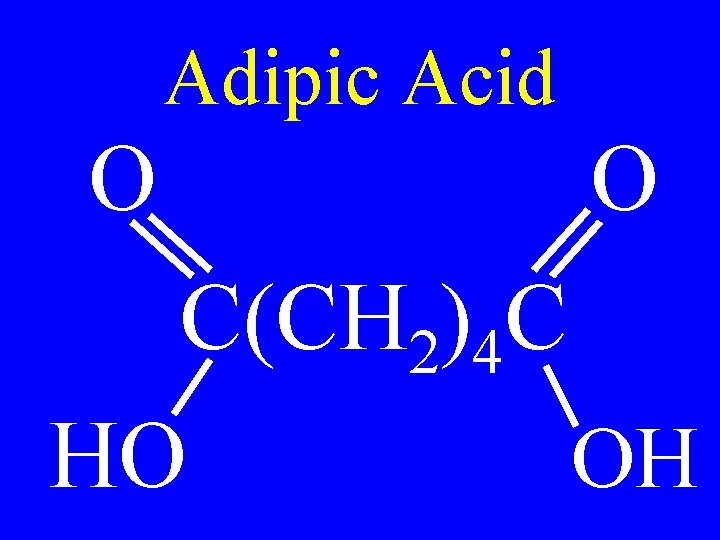
Adipic Acid O O C(CH 2)4 C HO OH
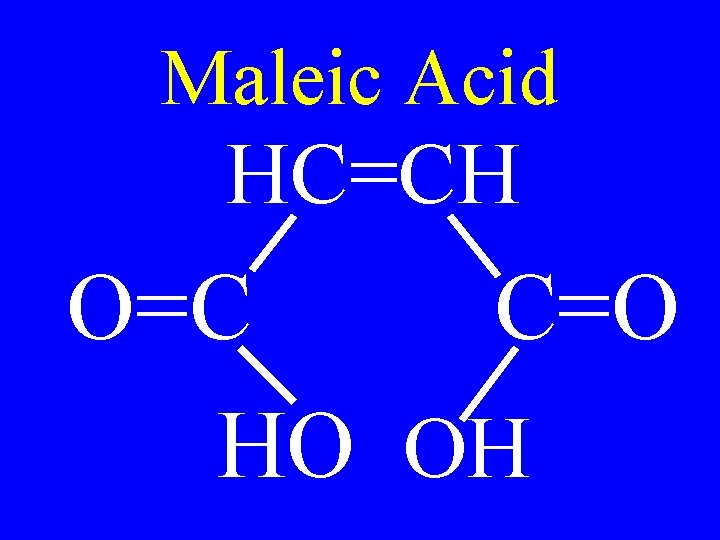
Maleic Acid HC=CH O=C C=O HO OH
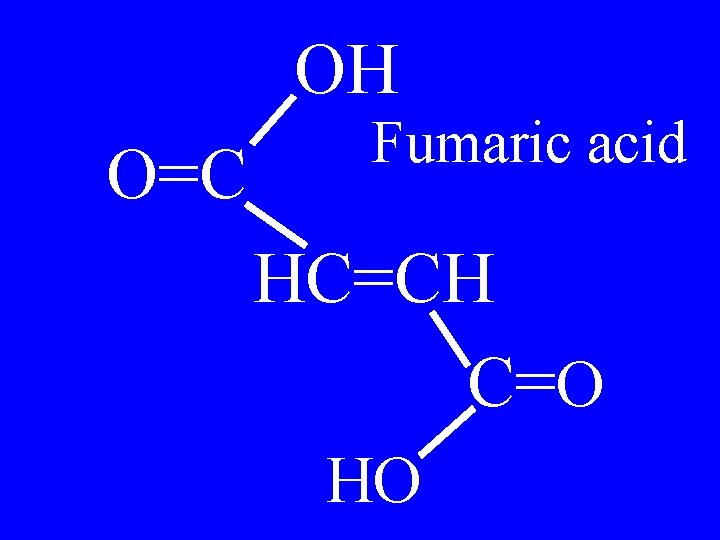
OH O=C Fumaric acid HC=CH C=O HO
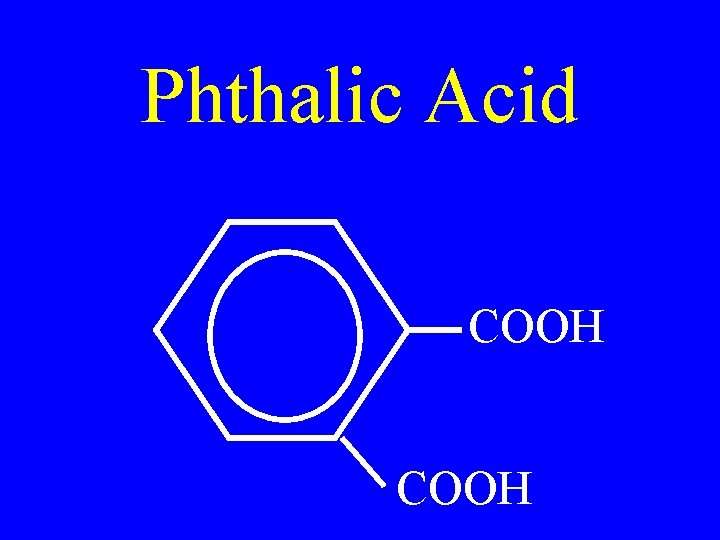
Phthalic Acid COOH
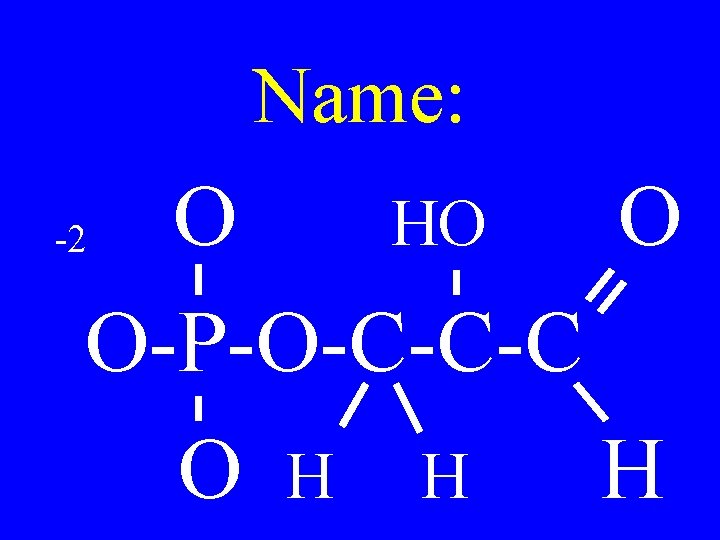
Name: O HO O O-P-O-C-C-C O H H H -2

Lactic Acid OH H 3 C-CH-COOH

Pyruvic Acid O H 3 C-C-COOH
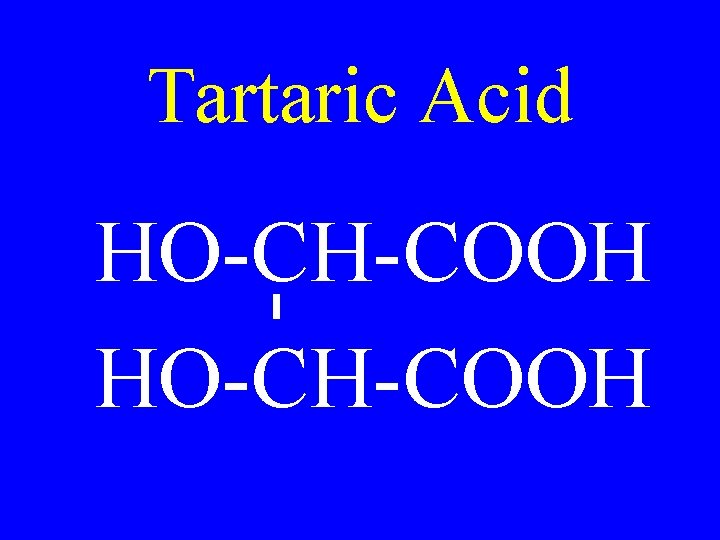
Tartaric Acid HO-CH-COOH
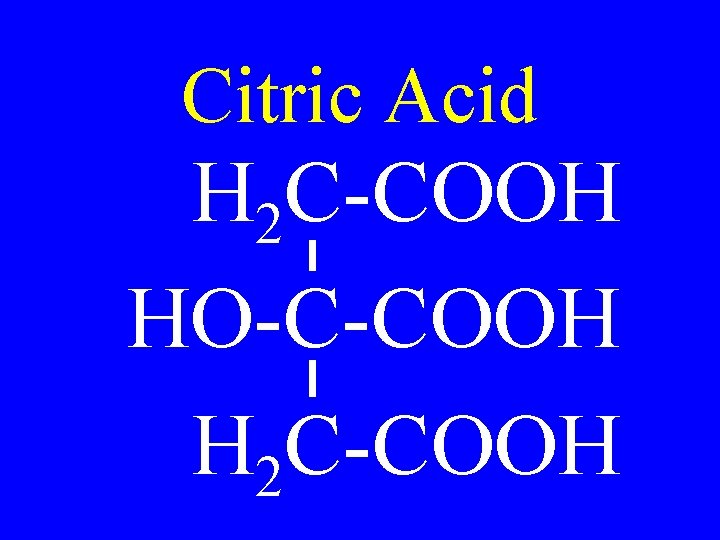
Citric Acid H 2 C-COOH HO-C-COOH H 2 C-COOH
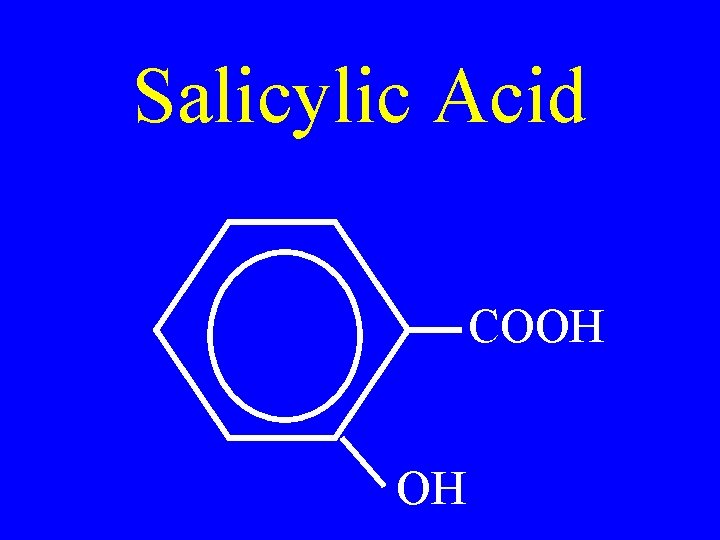
Salicylic Acid COOH OH

BP Properties • Alkanes < ketones • ketones < aldehydes • aldehydes < alcohols • alcohols < acids

Fatty Acids • Long chain fatty acids have a polar and a non-polar end
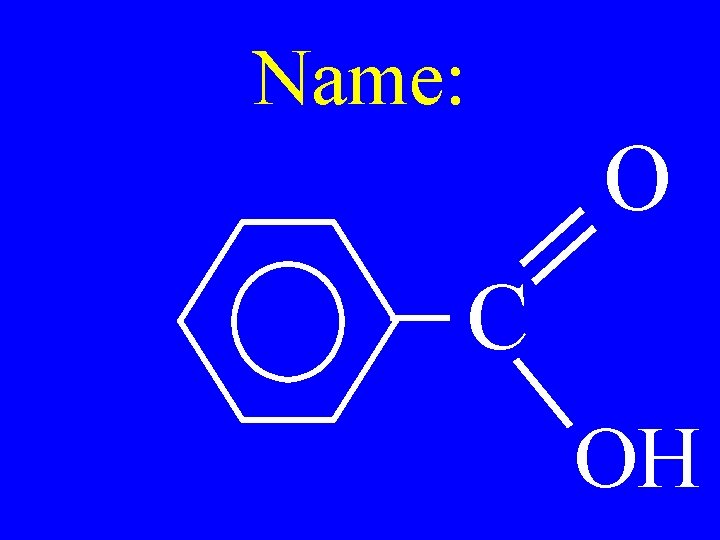
Name: O C OH
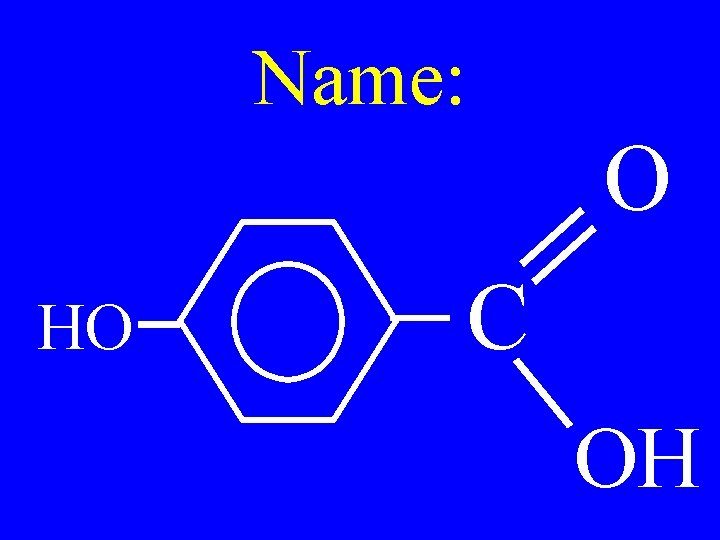
Name: O HO C OH

Drill: • Draw & name 4 carboxyl group containing isomers of C 5 H 10 O 2:
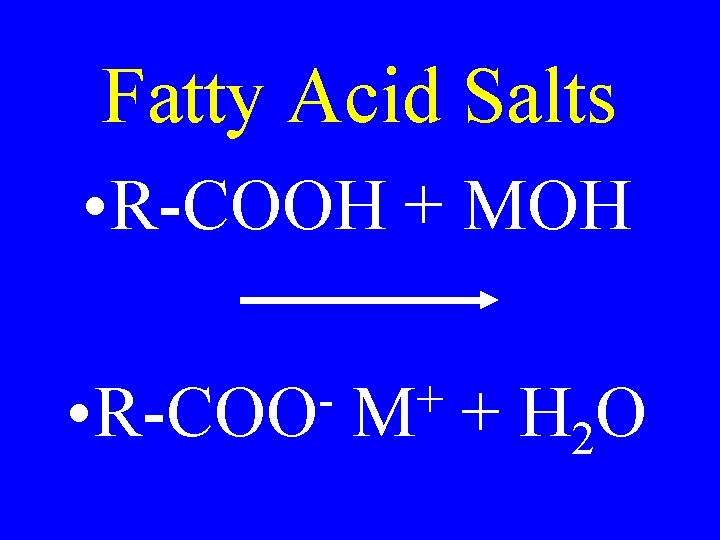
Fatty Acid Salts • R-COOH + MOH • R-COO + M + H 2 O
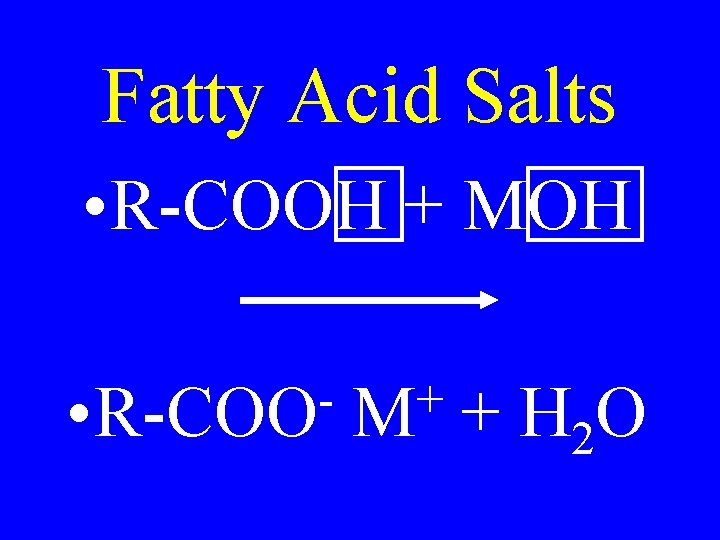
Fatty Acid Salts • R-COOH + MOH • R-COO + M + H 2 O
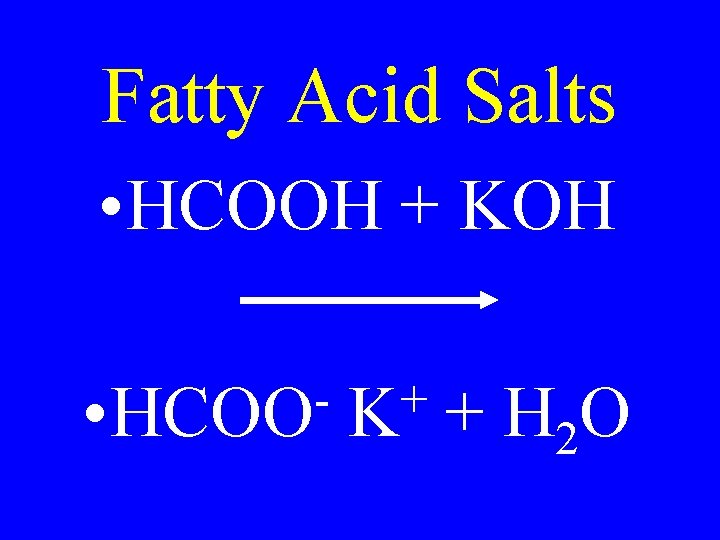
Fatty Acid Salts • HCOOH + KOH • HCOO + K + H 2 O
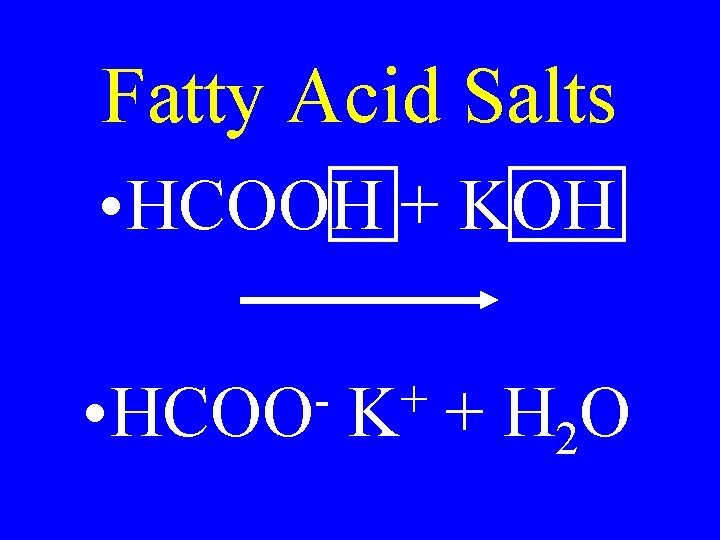
Fatty Acid Salts • HCOOH + KOH • HCOO + K + H 2 O
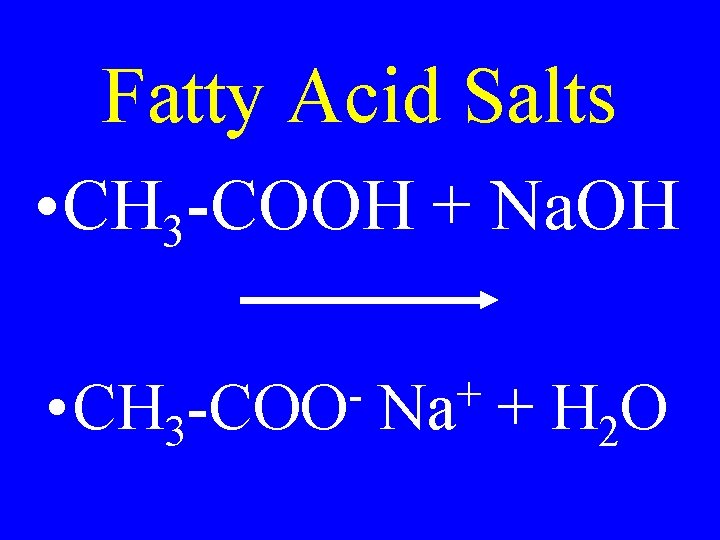
Fatty Acid Salts • CH 3 -COOH + Na. OH • CH 3 -COO + Na + H 2 O

Soaps • Soaps are alkali metal salts of long chain fatty acids

Soaps Hydrophobic end O O- Hydrophilic end

Explain Soaps, Micelles, & lipid layers

Making Carboxylic Acids

Making Acids • Strong oxidation of a primary alcohol or an aldehyde • Use KMn. O 4 or K 2 Cr 2 O 7
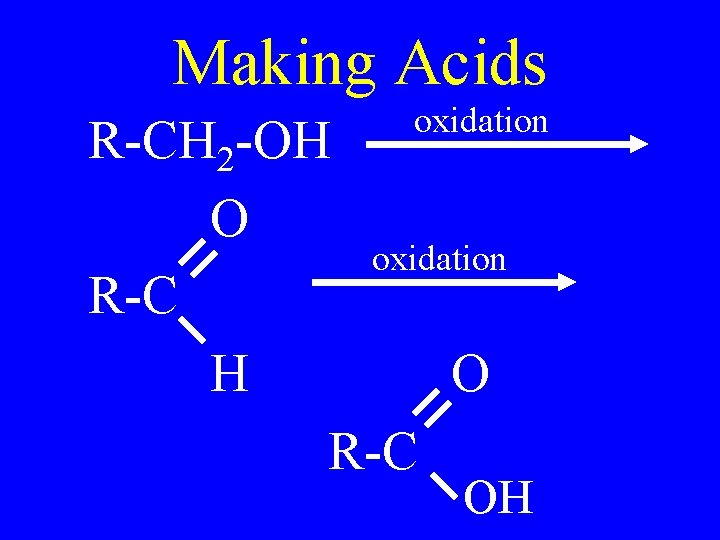
Making Acids oxidation R-CH 2 -OH O oxidation R-C H O R-C OH
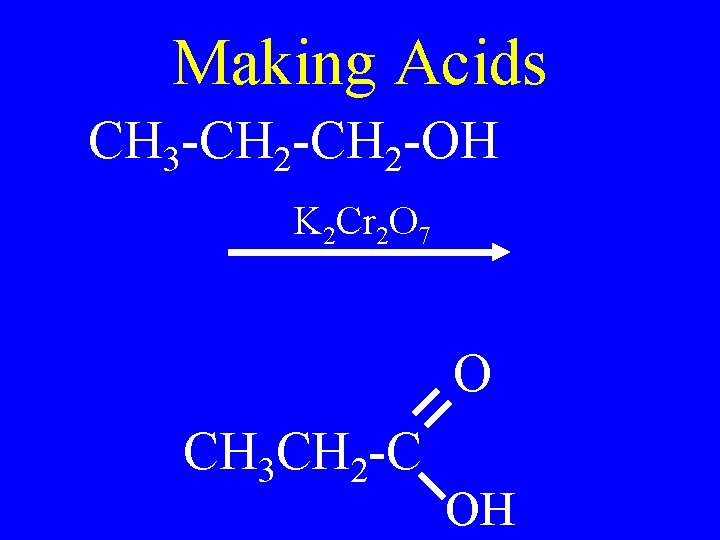
Making Acids CH 3 -CH 2 -OH K 2 Cr 2 O 7 O CH 3 CH 2 -C OH
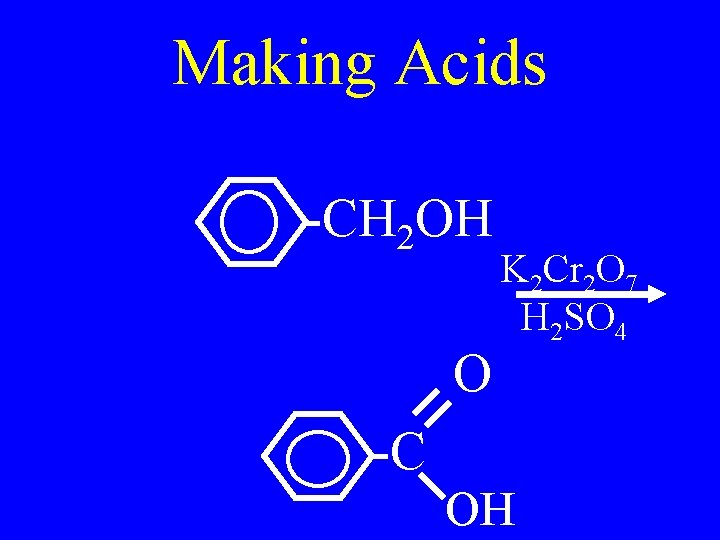
Making Acids -CH 2 OH O K 2 Cr 2 O 7 H 2 SO 4 -C OH
- Slides: 51