CARBOXYLIC ACIDS CARBOXYLIC ACIDS and their derivatives Carboxylic

CARBOXYLIC ACIDS
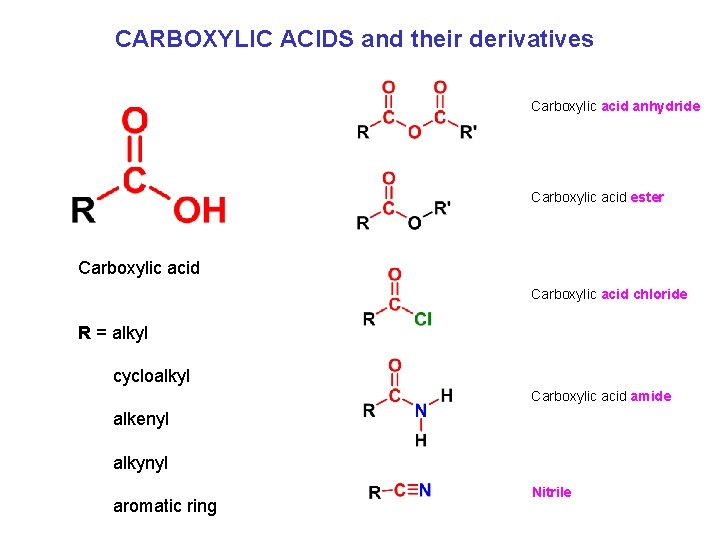
CARBOXYLIC ACIDS and their derivatives Carboxylic acid anhydride Carboxylic acid ester Carboxylic acid chloride R = alkyl cycloalkyl Carboxylic acid amide alkenyl alkynyl aromatic ring Nitrile
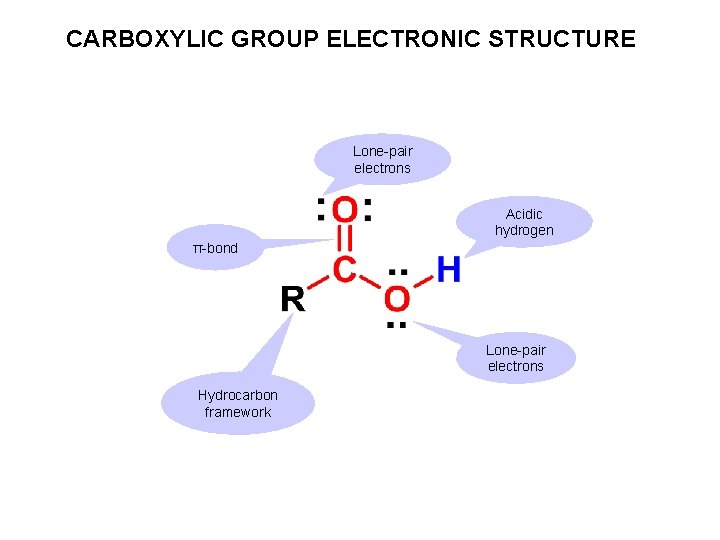
CARBOXYLIC GROUP ELECTRONIC STRUCTURE Lone-pair electrons Acidic hydrogen π-bond Lone-pair electrons Hydrocarbon framework
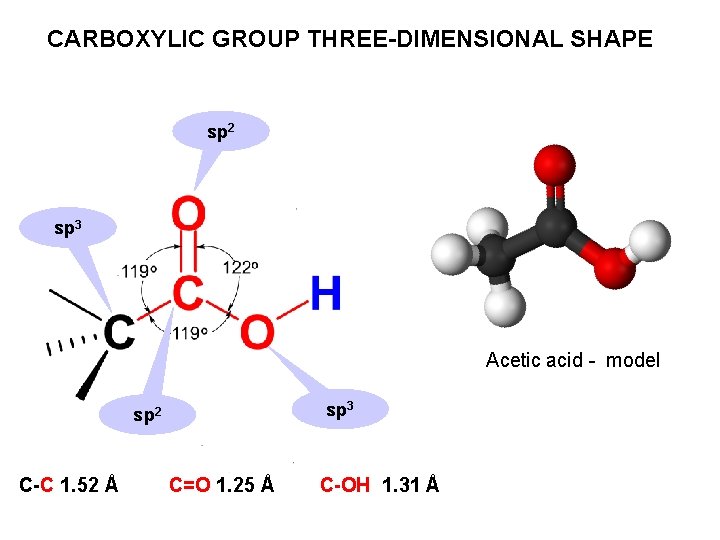
CARBOXYLIC GROUP THREE-DIMENSIONAL SHAPE sp 2 sp 3 Acetic acid - model sp 3 sp 2 C-C 1. 52 Å C=O 1. 25 Å C-OH 1. 31 Å
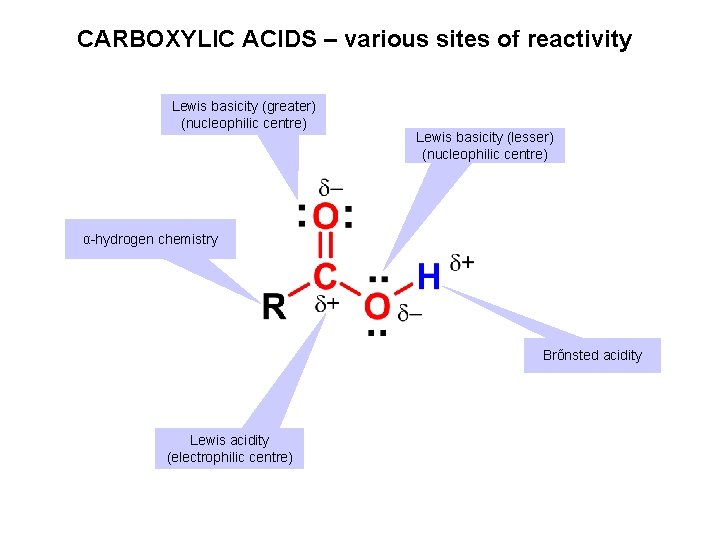
CARBOXYLIC ACIDS – various sites of reactivity Lewis basicity (greater) (nucleophilic centre) Lewis basicity (lesser) (nucleophilic centre) α-hydrogen chemistry Brőnsted acidity Lewis acidity (electrophilic centre)
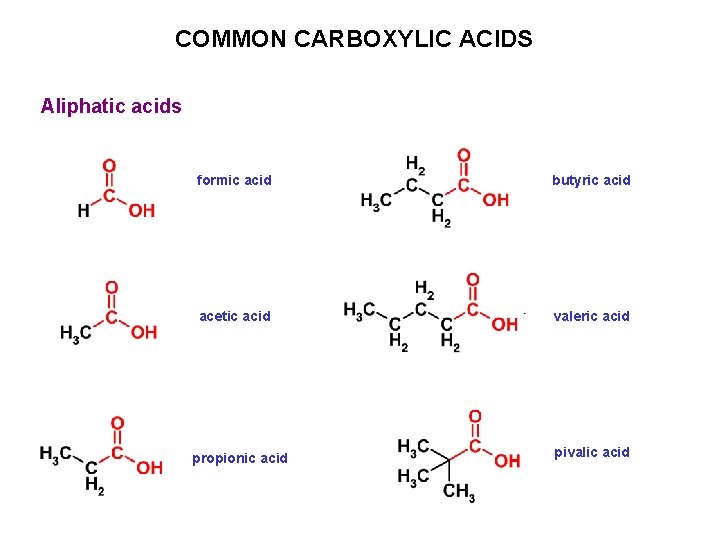
COMMON CARBOXYLIC ACIDS Aliphatic acids formic acid butyric acid acetic acid valeric acid propionic acid pivalic acid
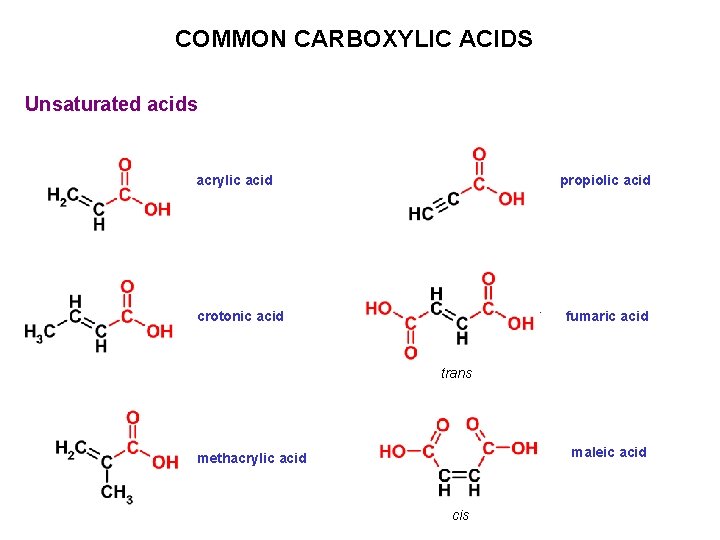
COMMON CARBOXYLIC ACIDS Unsaturated acids acrylic acid propiolic acid crotonic acid fumaric acid trans maleic acid methacrylic acid cis
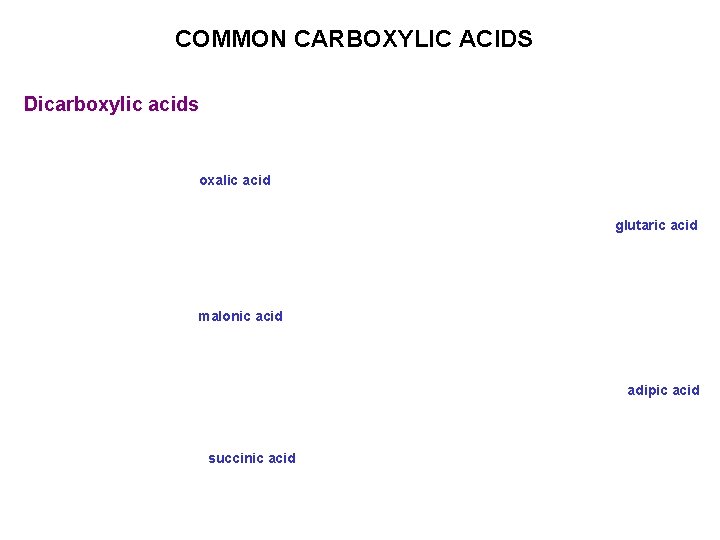
COMMON CARBOXYLIC ACIDS Dicarboxylic acids oxalic acid glutaric acid malonic acid adipic acid succinic acid
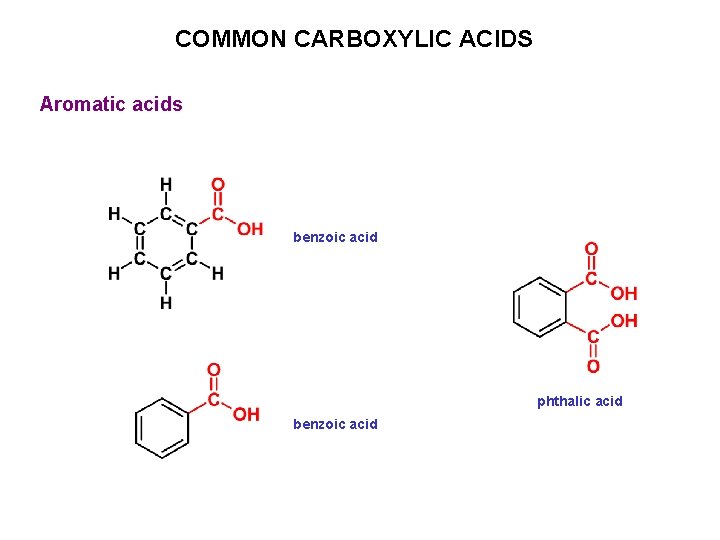
COMMON CARBOXYLIC ACIDS Aromatic acids benzoic acid phthalic acid benzoic acid
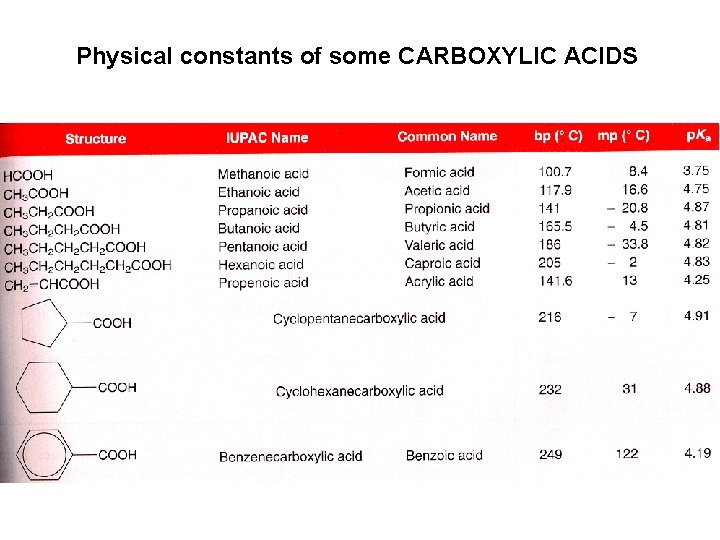
Physical constants of some CARBOXYLIC ACIDS
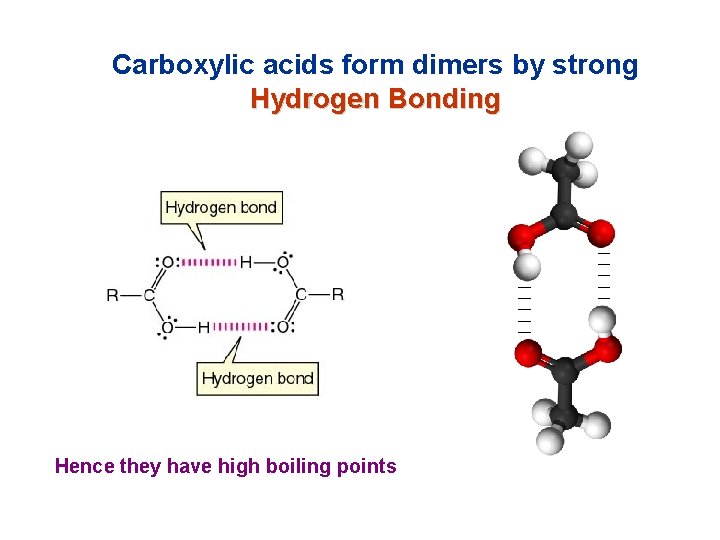
Carboxylic acids form dimers by strong Hydrogen Bonding Hence they have high boiling points
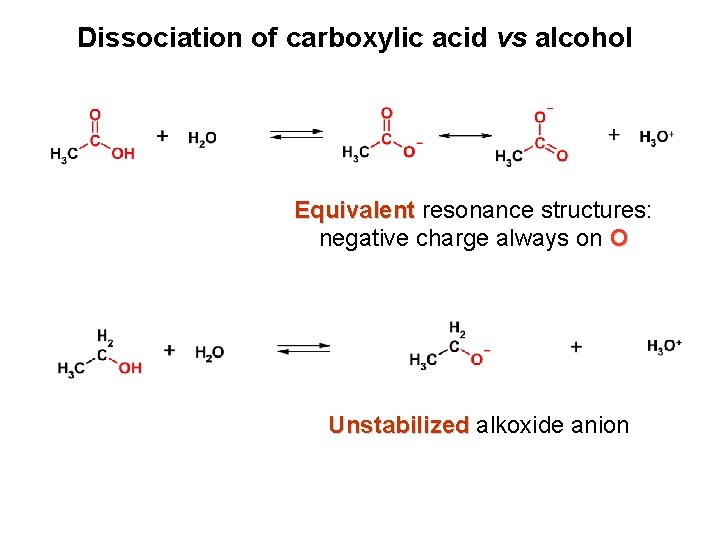
Dissociation of carboxylic acid vs alcohol Equivalent resonance structures: negative charge always on O Unstabilized alkoxide anion
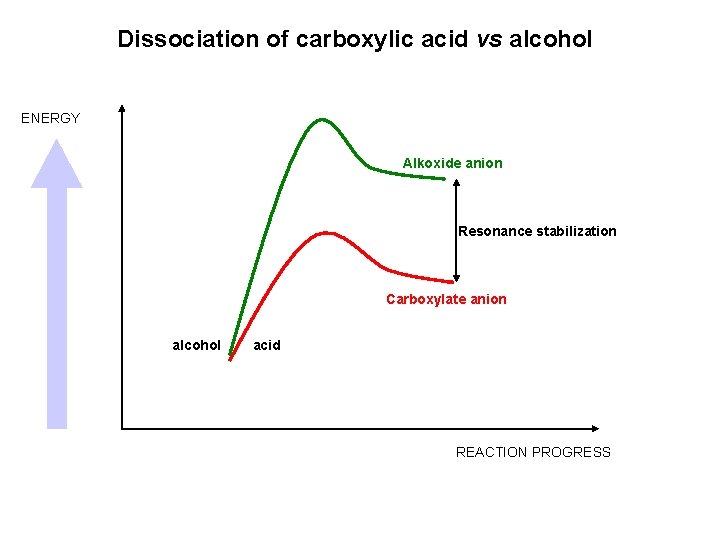
Dissociation of carboxylic acid vs alcohol ENERGY Alkoxide anion Resonance stabilization Carboxylate anion alcohol acid REACTION PROGRESS
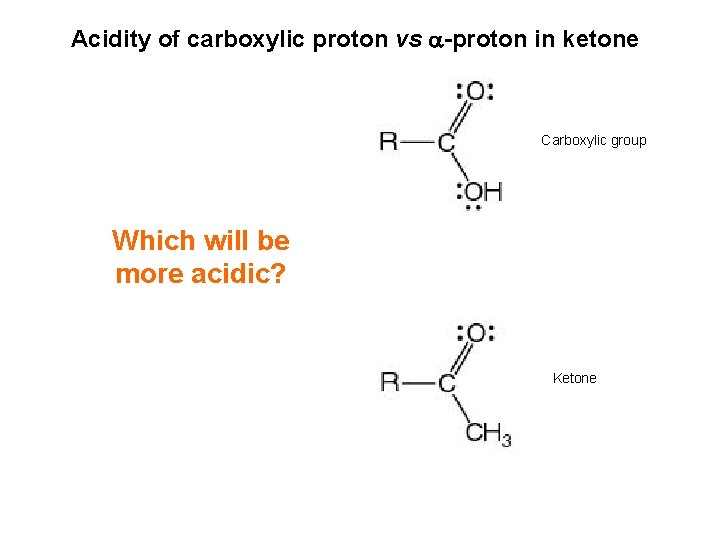
Acidity of carboxylic proton vs -proton in ketone Carboxylic group Which will be more acidic? Ketone
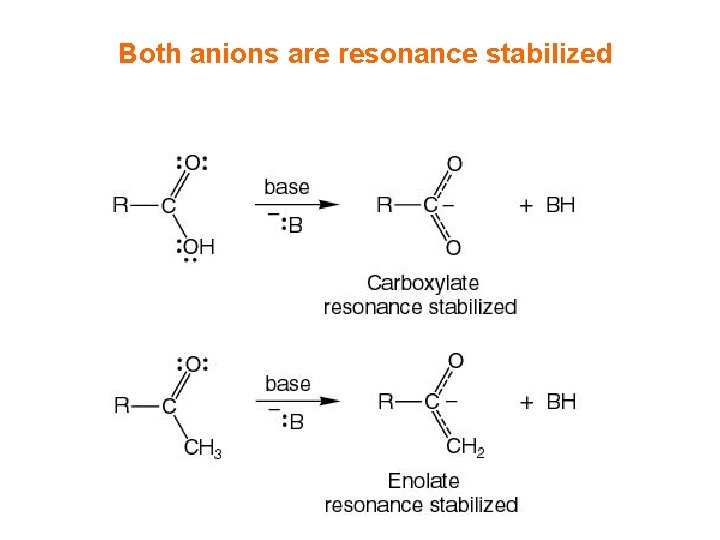
Both anions are resonance stabilized
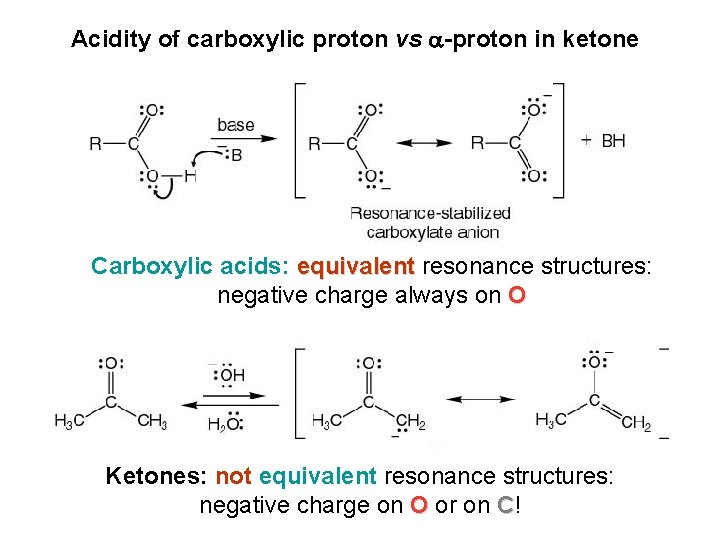
Acidity of carboxylic proton vs -proton in ketone Carboxylic acids: equivalent resonance structures: negative charge always on O Ketones: not equivalent resonance structures: negative charge on O or on C!
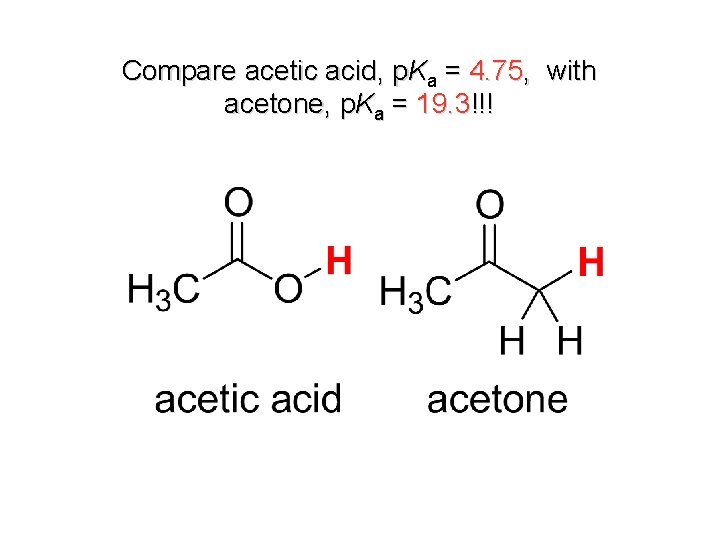
Compare acetic acid, p. Ka = 4. 75, with acetone, p. Ka = 19. 3!!!
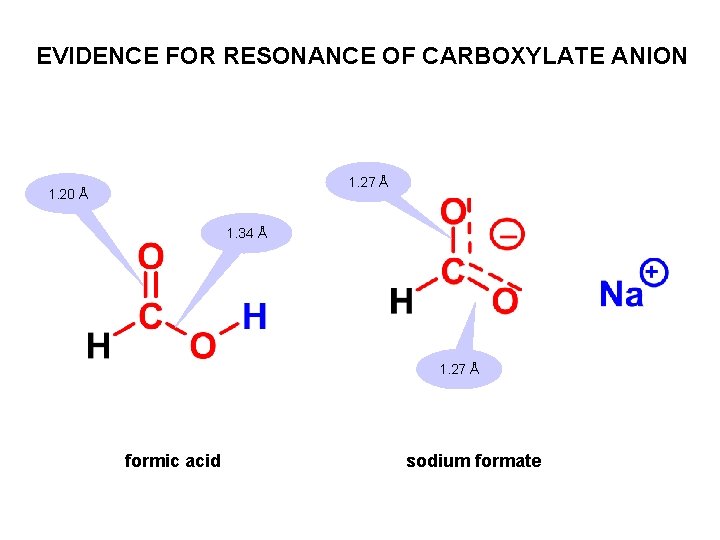
EVIDENCE FOR RESONANCE OF CARBOXYLATE ANION 1. 27 Å 1. 20 Å 1. 34 Å 1. 27 Å formic acid sodium formate
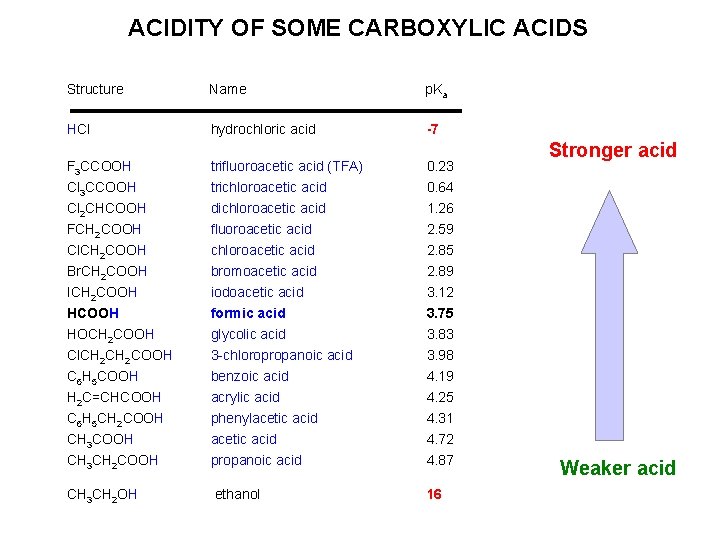
ACIDITY OF SOME CARBOXYLIC ACIDS Structure Name p. Ka HCl hydrochloric acid -7 F 3 CCOOH Cl 2 CHCOOH FCH 2 COOH trifluoroacetic acid (TFA) trichloroacetic acid dichloroacetic acid fluoroacetic acid 0. 23 0. 64 1. 26 2. 59 Cl. CH 2 COOH chloroacetic acid 2. 85 Br. CH 2 COOH bromoacetic acid 2. 89 ICH 2 COOH iodoacetic acid 3. 12 HCOOH formic acid 3. 75 HOCH 2 COOH glycolic acid 3. 83 Cl. CH 2 COOH C 6 H 5 COOH H 2 C=CHCOOH C 6 H 5 CH 2 COOH CH 3 CH 2 COOH 3 -chloropropanoic acid benzoic acid acrylic acid phenylacetic acid propanoic acid 3. 98 4. 19 4. 25 4. 31 4. 72 4. 87 CH 3 CH 2 OH ethanol 16 Stronger acid Weaker acid
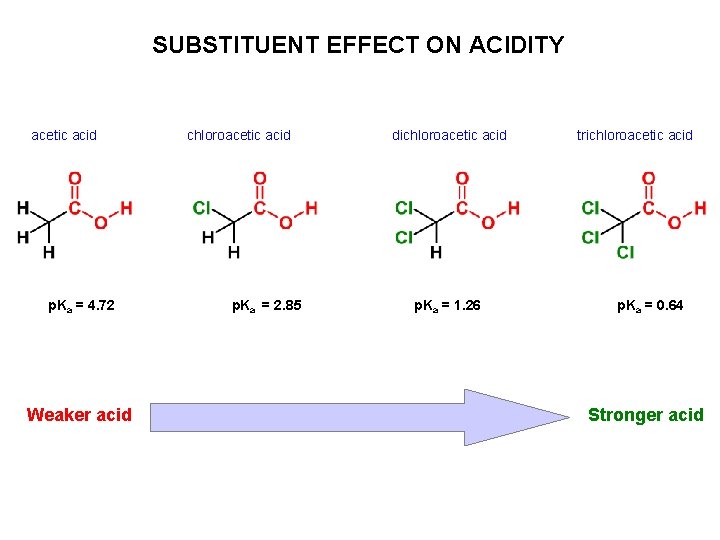
SUBSTITUENT EFFECT ON ACIDITY acetic acid p. Ka = 4. 72 Weaker acid chloroacetic acid p. Ka = 2. 85 dichloroacetic acid p. Ka = 1. 26 trichloroacetic acid p. Ka = 0. 64 Stronger acid
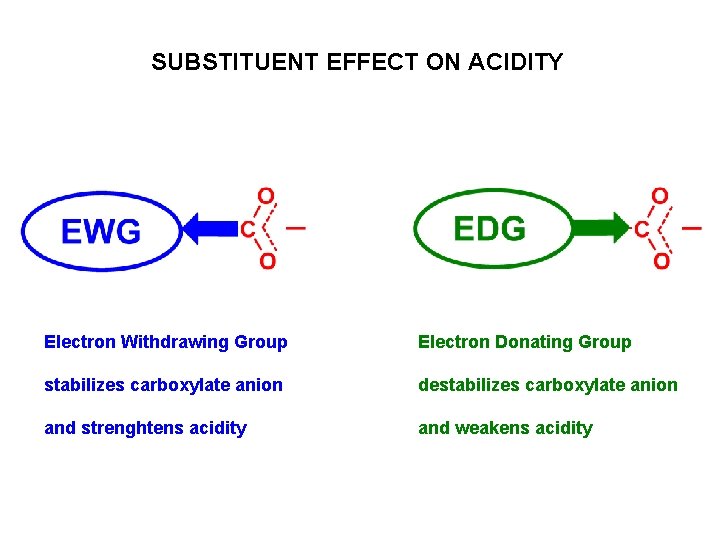
SUBSTITUENT EFFECT ON ACIDITY Electron Withdrawing Group Electron Donating Group stabilizes carboxylate anion destabilizes carboxylate anion and strenghtens acidity and weakens acidity
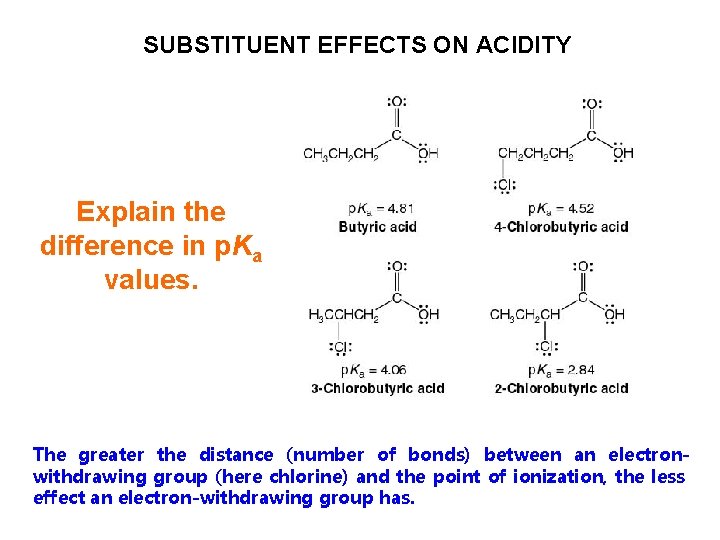
SUBSTITUENT EFFECTS ON ACIDITY Explain the difference in p. Ka values. The greater the distance (number of bonds) between an electronwithdrawing group (here chlorine) and the point of ionization, the less effect an electron-withdrawing group has.
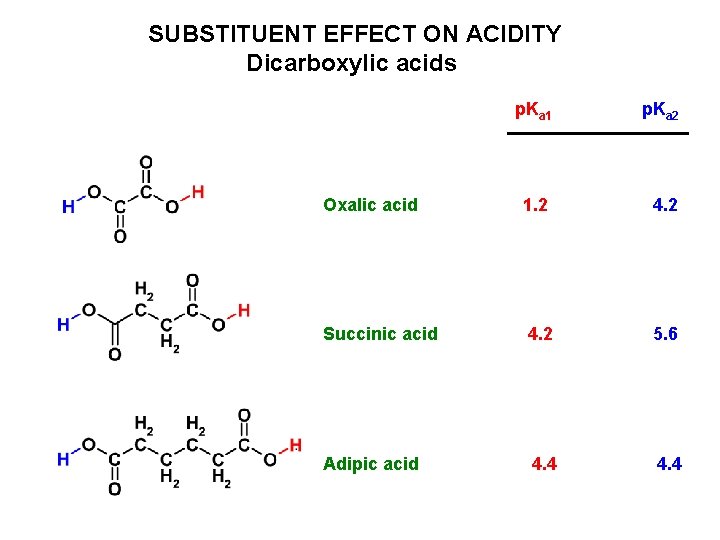
SUBSTITUENT EFFECT ON ACIDITY Dicarboxylic acids p. Ka 1 p. Ka 2 Oxalic acid 1. 2 4. 2 Succinic acid 4. 2 5. 6 Adipic acid 4. 4
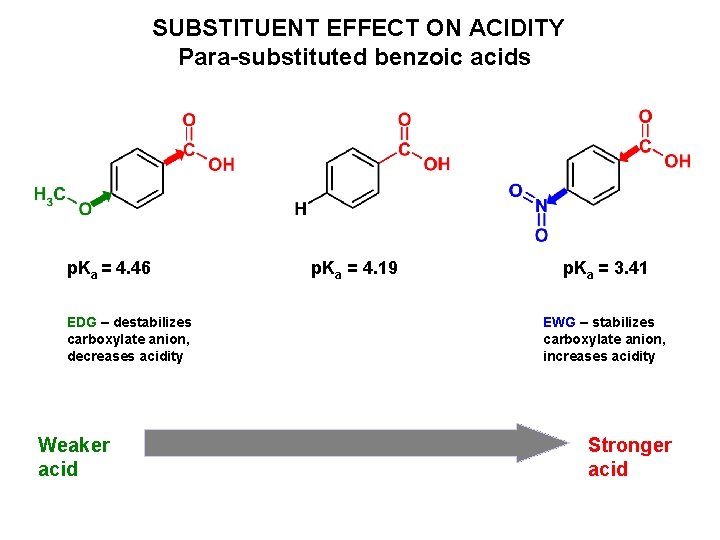
SUBSTITUENT EFFECT ON ACIDITY Para-substituted benzoic acids p. Ka = 4. 46 EDG – destabilizes carboxylate anion, decreases acidity Weaker acid p. Ka = 4. 19 p. Ka = 3. 41 EWG – stabilizes carboxylate anion, increases acidity Stronger acid
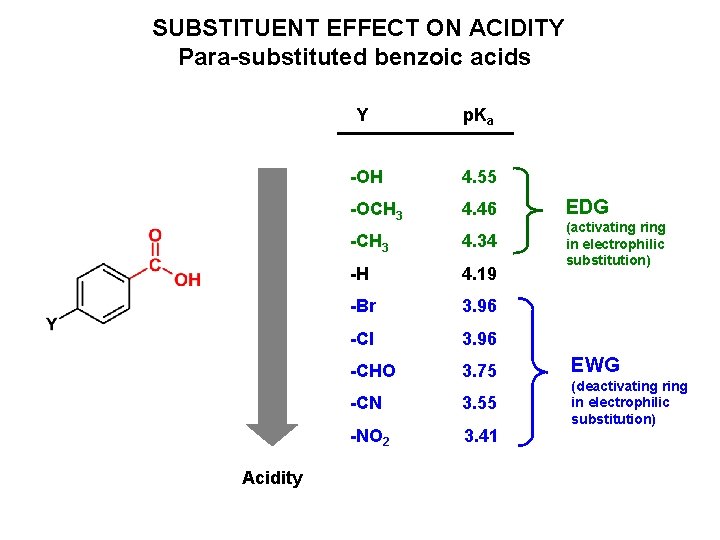
SUBSTITUENT EFFECT ON ACIDITY Para-substituted benzoic acids Acidity Y p. Ka -OH 4. 55 -OCH 3 4. 46 EDG -CH 3 4. 34 -H 4. 19 (activating ring in electrophilic substitution) -Br 3. 96 -Cl 3. 96 -CHO 3. 75 -CN 3. 55 -NO 2 3. 41 EWG (deactivating ring in electrophilic substitution)

Preparation of carboxylic acids (Industrial methods)
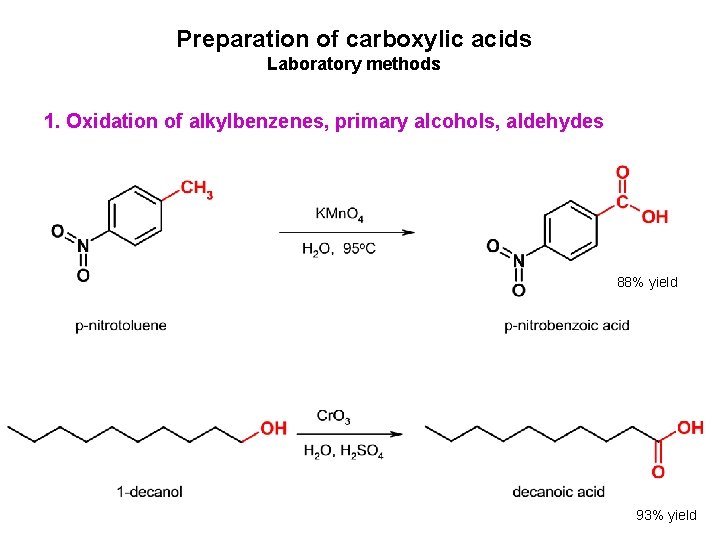
Preparation of carboxylic acids Laboratory methods 1. Oxidation of alkylbenzenes, primary alcohols, aldehydes 88% yield 93% yield
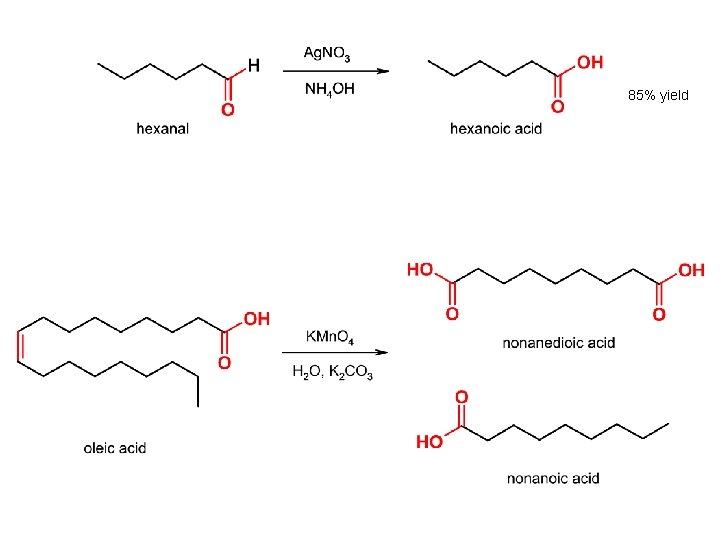
85% yield
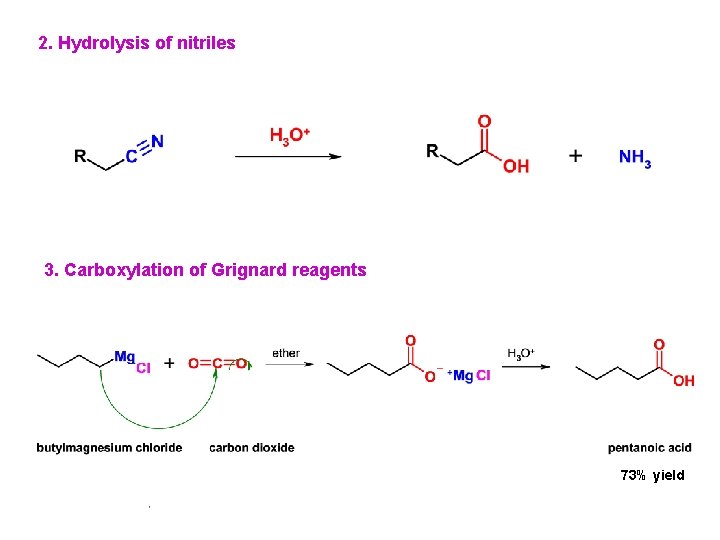
2. Hydrolysis of nitriles 3. Carboxylation of Grignard reagents 73% yield
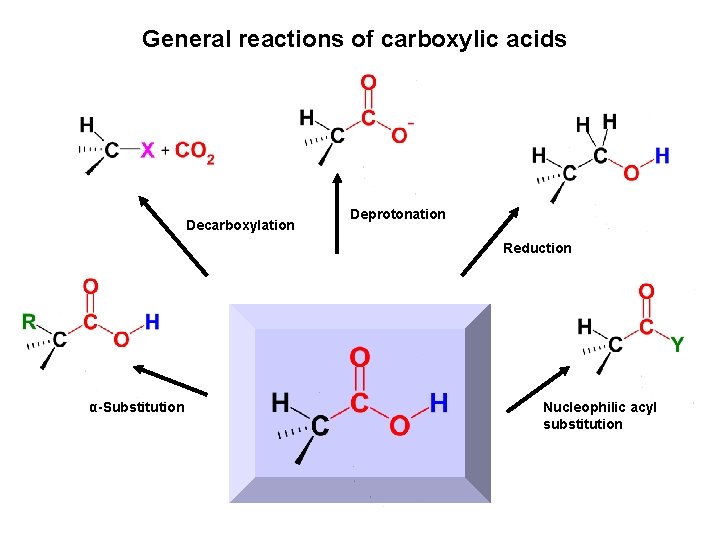
General reactions of carboxylic acids Decarboxylation Deprotonation Reduction α-Substitution Nucleophilic acyl substitution
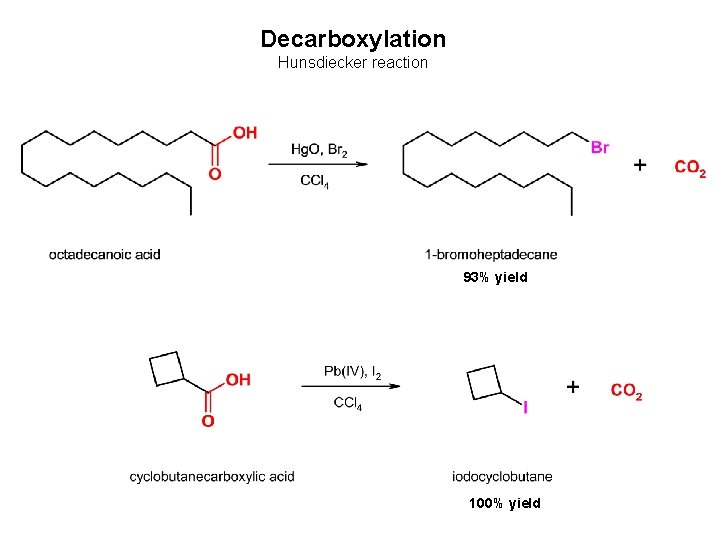
Decarboxylation Hunsdiecker reaction 93% yield 100% yield
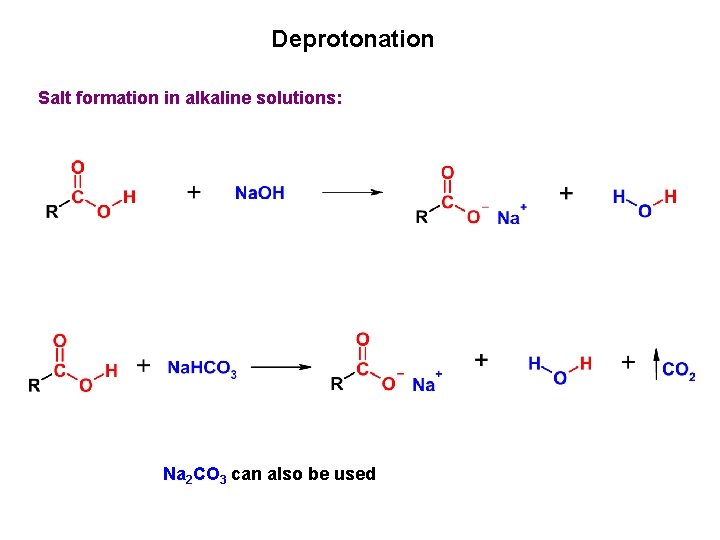
Deprotonation Salt formation in alkaline solutions: Na 2 CO 3 can also be used
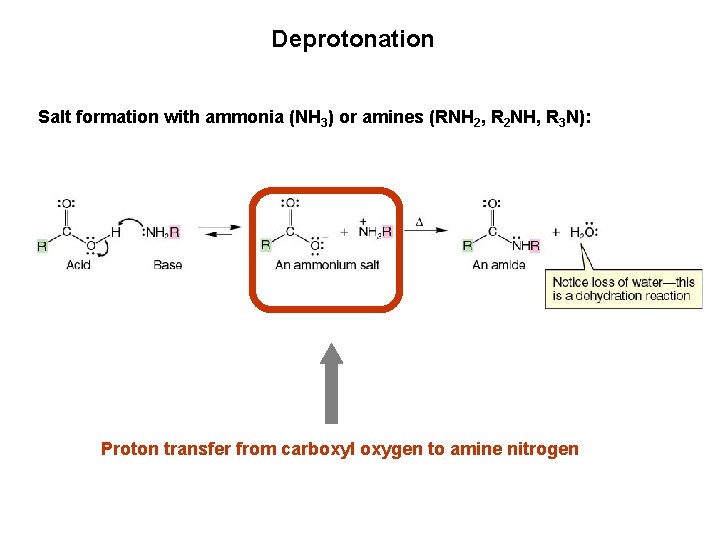
Deprotonation Salt formation with ammonia (NH 3) or amines (RNH 2, R 2 NH, R 3 N): Proton transfer from carboxyl oxygen to amine nitrogen
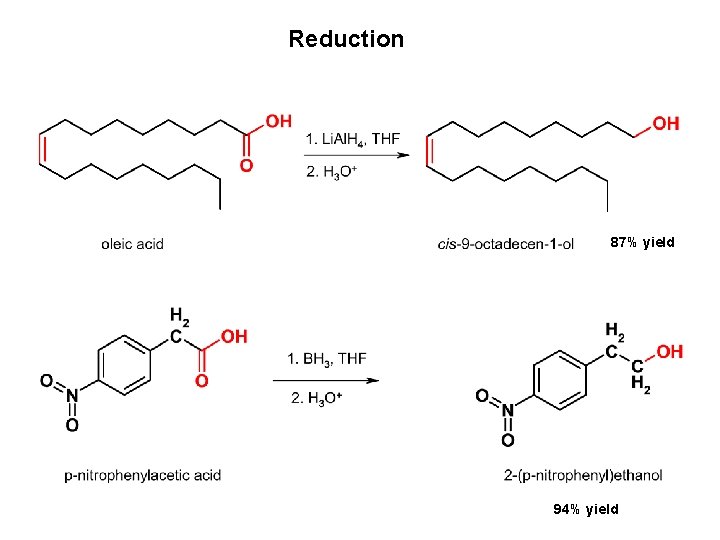
Reduction 87% yield 94% yield
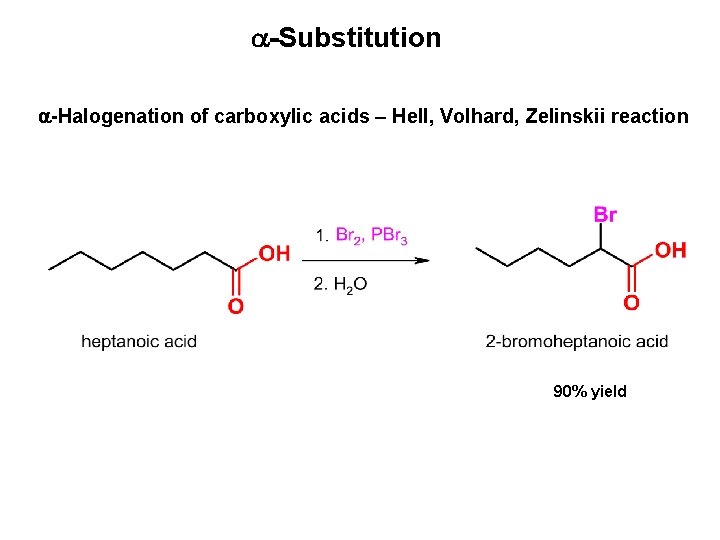
-Substitution -Halogenation of carboxylic acids – Hell, Volhard, Zelinskii reaction 90% yield

Mechanism of -Halogenation
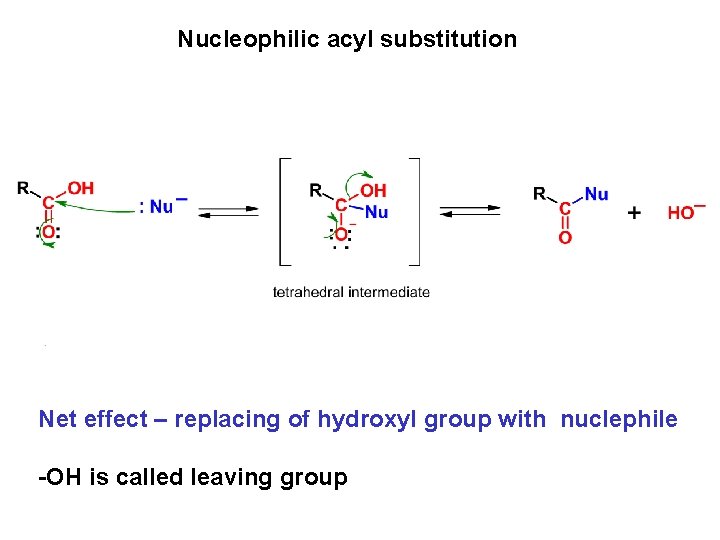
Nucleophilic acyl substitution Net effect – replacing of hydroxyl group with nuclephile -OH is called leaving group
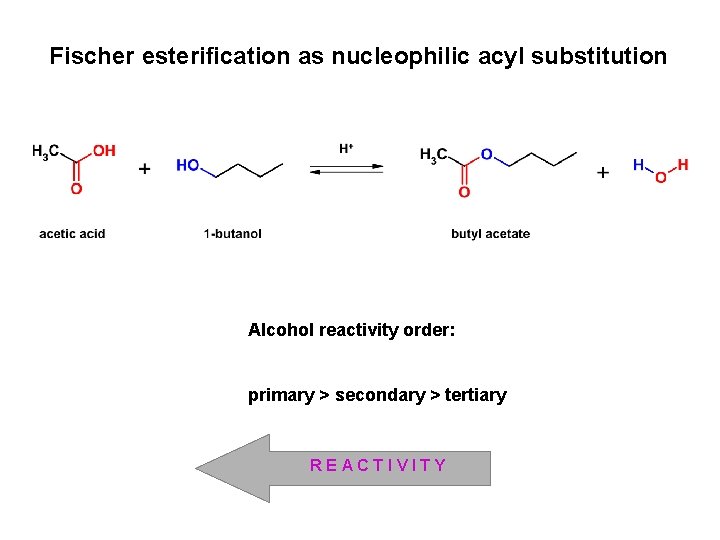
Fischer esterification as nucleophilic acyl substitution Alcohol reactivity order: primary > secondary > tertiary REACTIVITY
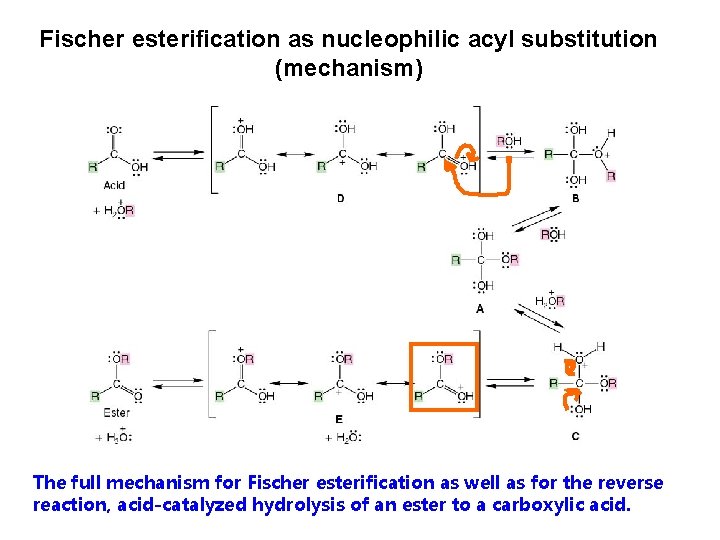
Fischer esterification as nucleophilic acyl substitution (mechanism) The full mechanism for Fischer esterification as well as for the reverse reaction, acid-catalyzed hydrolysis of an ester to a carboxylic acid.
- Slides: 39