Carboxylic Acids and Esters Section 1 6 Carboxylic

Carboxylic Acids and Esters Section 1. 6

Carboxylic Acids n Carboxylic Acid – an organic compound that contains a carboxyl group (COOH) Can easily lose a hydrogen ion when dissolved in an aqueous solution making the solution slightly acidic n Citrus fruits, crabapples, rhubarb and other foods that contain carboxylic acids have a sour, tangy taste n
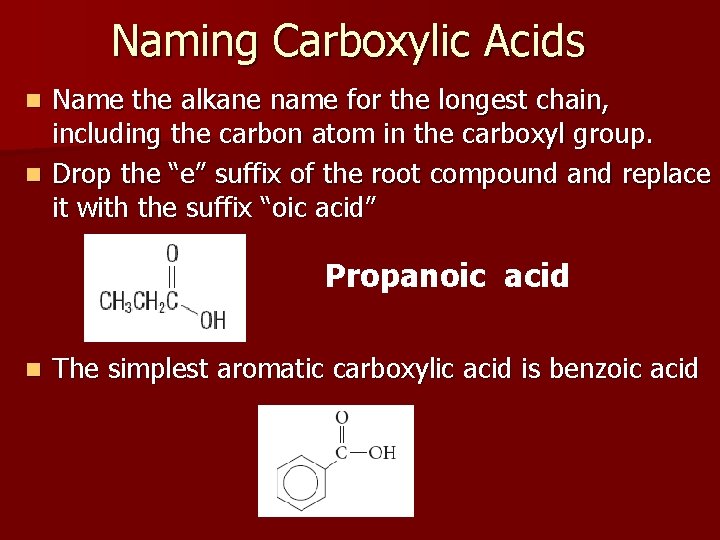
Naming Carboxylic Acids Name the alkane name for the longest chain, including the carbon atom in the carboxyl group. n Drop the “e” suffix of the root compound and replace it with the suffix “oic acid” n Propanoic acid n The simplest aromatic carboxylic acid is benzoic acid
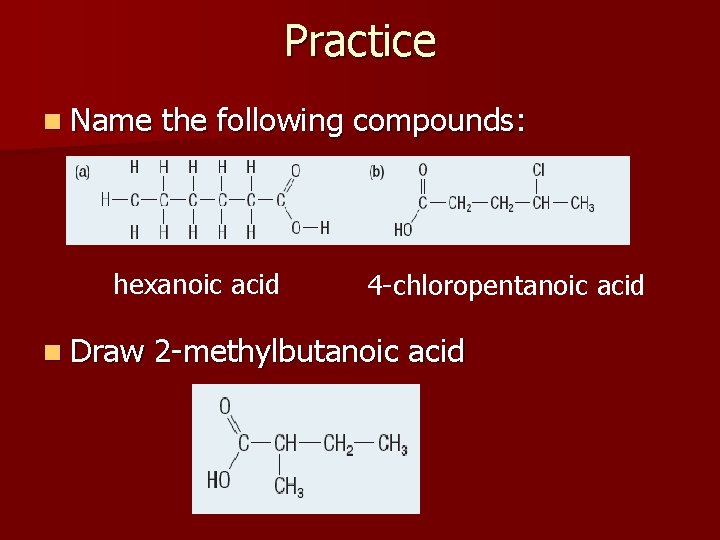
Practice n Name the following compounds: hexanoic acid n Draw 4 -chloropentanoic acid 2 -methylbutanoic acid

Properties of Carboxylic Acids n Since carbonxylic acids have 2 polar groups – carbonyl and a hydroxyl located close together, their molecules are very polar. The hydroxyl groups form hydrogen bonds with other molecules, making them very soluble in water. n Larger carboxylic acids have decreasing solubility due to the large non-polar chain n

Properties continued… n Carboxylic acids share many properties with other acids n Can be detected by litmus paper n Carboxylic acids also react with bases to form ionic compounds and water. n The melting points of carboxylic acids are higher than their hydrocarbon counterparts because of their polar parts

Esters n Ester – an organic compound that contains a carbonyl group bonded to a second oxygen atom which is bonded to another carbon atom Many plants naturally produce esters which are responsible for many of the odours of fruits, flowers and perfumes. n Synthetic esters are often used as flavourings in processed foods and as scents in cosmetics and perfumes. n
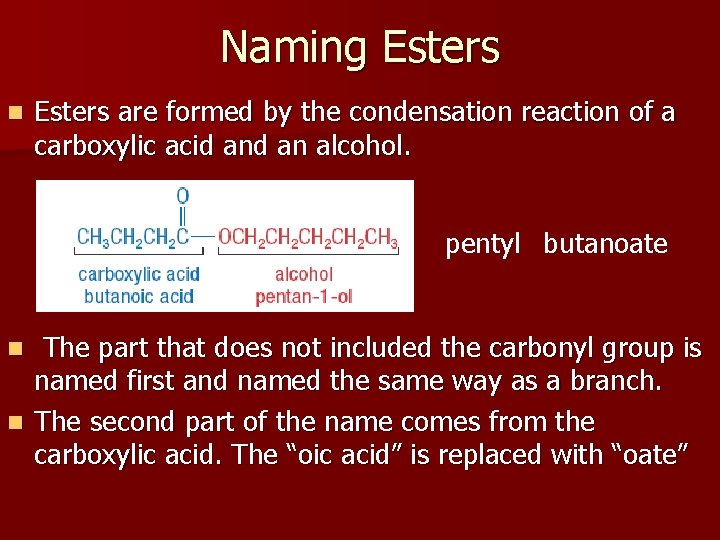
Naming Esters n Esters are formed by the condensation reaction of a carboxylic acid an alcohol. pentyl butanoate The part that does not included the carbonyl group is named first and named the same way as a branch. n The second part of the name comes from the carboxylic acid. The “oic acid” is replaced with “oate” n
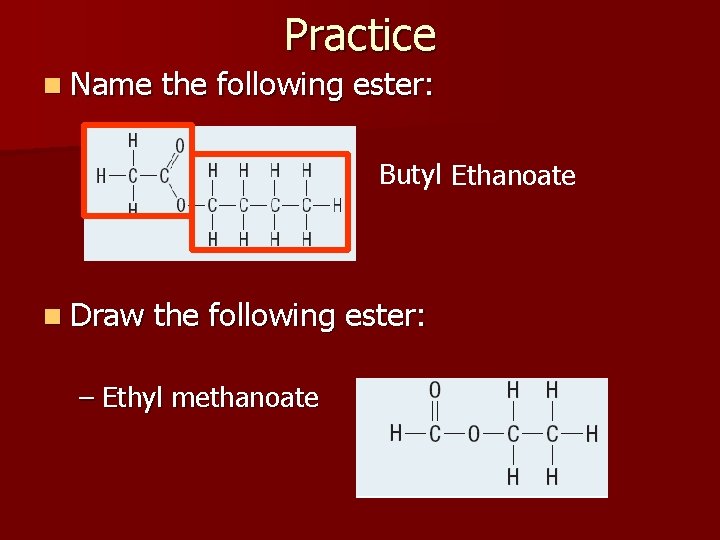
Practice n Name the following ester: Butyl Ethanoate n Draw the following ester: – Ethyl methanoate

Properties of Esters The functional group is similar to the carboxyl group of an acid, but without the hydroxyl group. As a result esters are less polar than carboxylic acids and do NOT form hydrogen bonding. n Small esters are soluble in water due to the polarity of the C-O bonds. Esters are less soluble than Carboxylic Acids and have lower boiling points. n
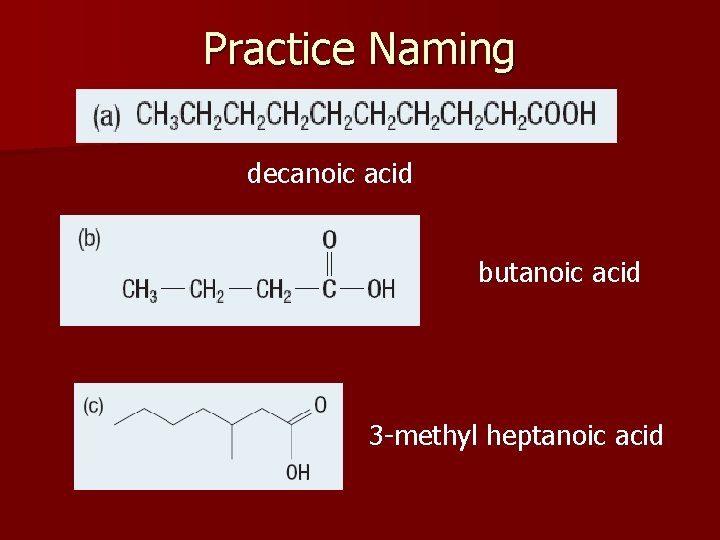
Practice Naming decanoic acid butanoic acid 3 -methyl heptanoic acid
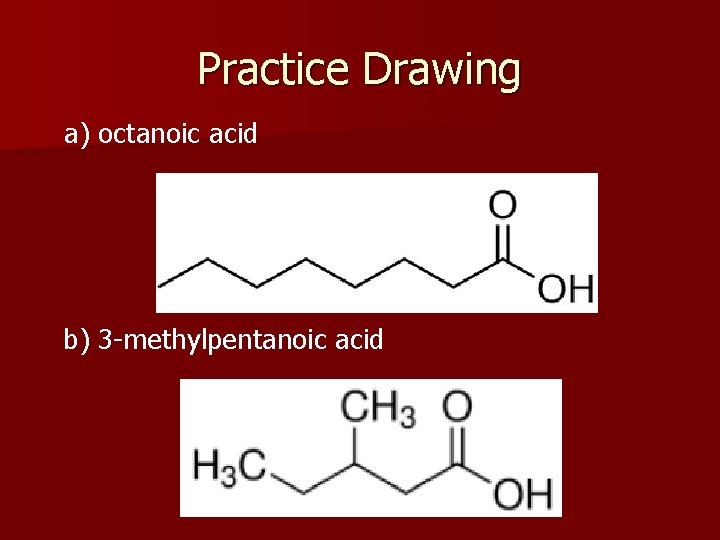
Practice Drawing a) octanoic acid b) 3 -methylpentanoic acid
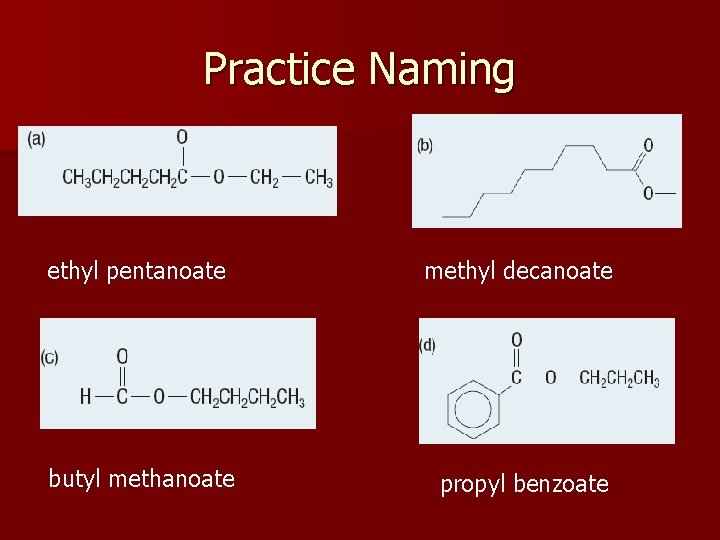
Practice Naming ethyl pentanoate methyl decanoate butyl methanoate propyl benzoate
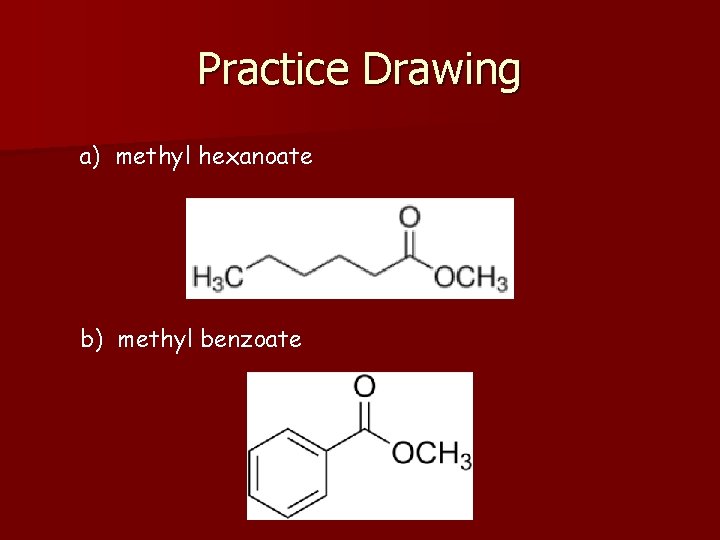
Practice Drawing a) methyl hexanoate b) methyl benzoate
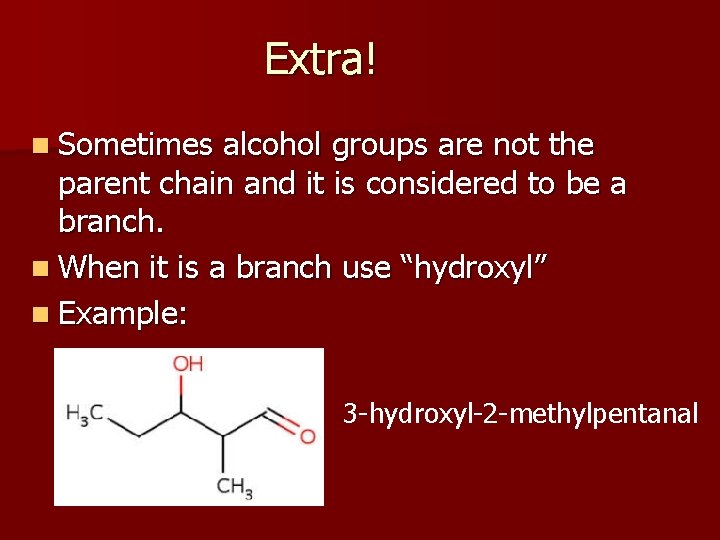
Extra! n Sometimes alcohol groups are not the parent chain and it is considered to be a branch. n When it is a branch use “hydroxyl” n Example: 3 -hydroxyl-2 -methylpentanal
- Slides: 15