CARBOXYLIC ACID DERIVATIVES CARBOXYLIC ACID An acyl group

CARBOXYLIC ACID DERIVATIVES
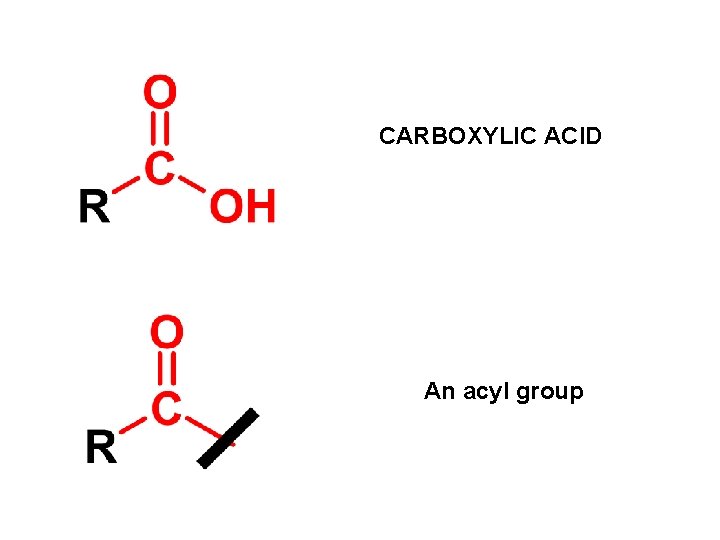
CARBOXYLIC ACID An acyl group
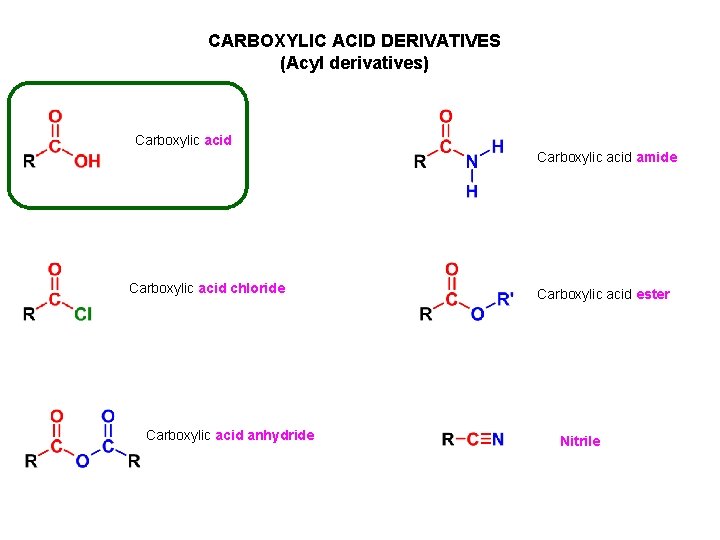
CARBOXYLIC ACID DERIVATIVES (Acyl derivatives) Carboxylic acid amide Carboxylic acid chloride Carboxylic acid anhydride Carboxylic acid ester Nitrile
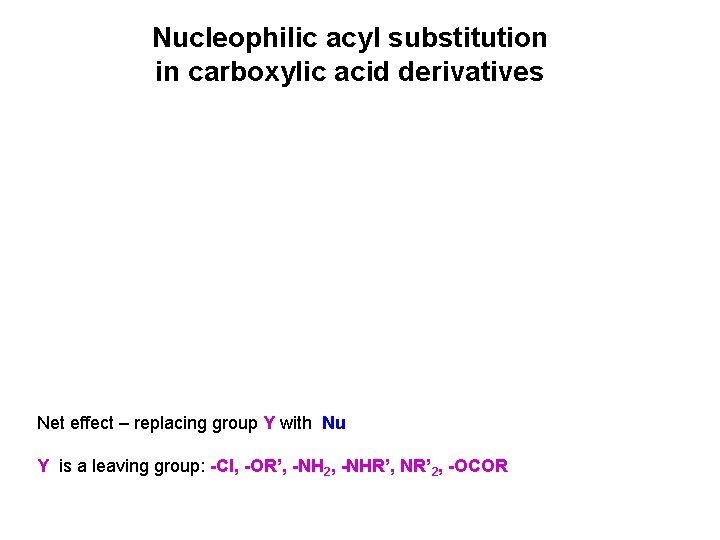
Nucleophilic acyl substitution in carboxylic acid derivatives Net effect – replacing group Y with Nu Y is a leaving group: -Cl, -OR’, -NH 2, -NHR’, NR’ 2, -OCOR
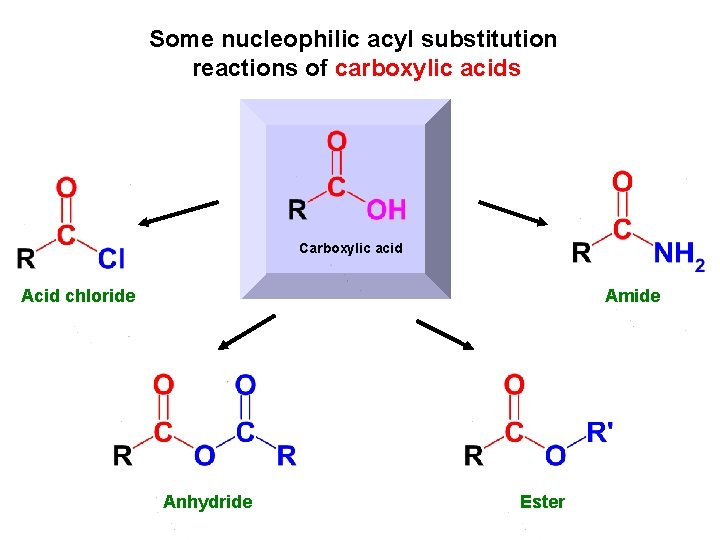
Some nucleophilic acyl substitution reactions of carboxylic acids Carboxylic acid Acid chloride Amide Anhydride Ester
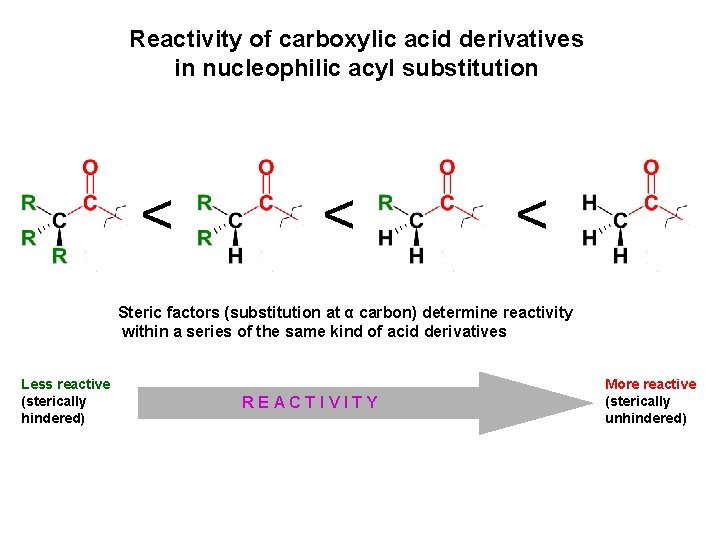
Reactivity of carboxylic acid derivatives in nucleophilic acyl substitution < < < Steric factors (substitution at α carbon) determine reactivity within a series of the same kind of acid derivatives Less reactive (sterically hindered) < REACTIVITY More reactive (sterically unhindered)
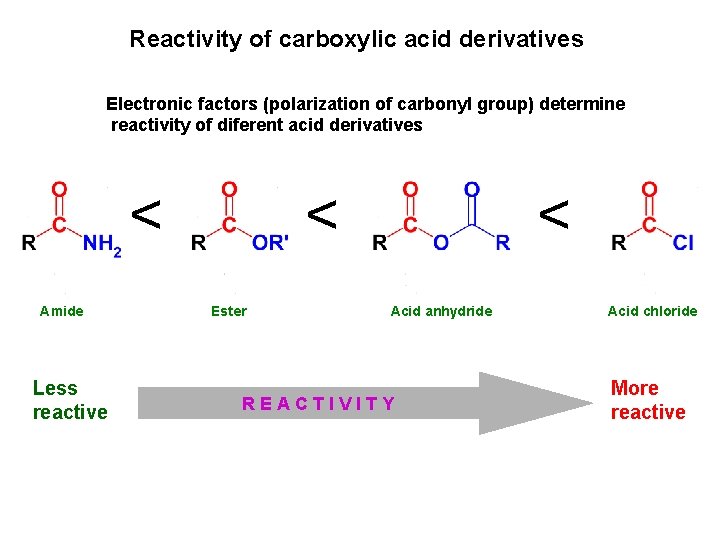
Reactivity of carboxylic acid derivatives Electronic factors (polarization of carbonyl group) determine reactivity of diferent acid derivatives < Amide Less reactive < Ester < < Acid anhydride REACTIVITY Acid chloride More reactive
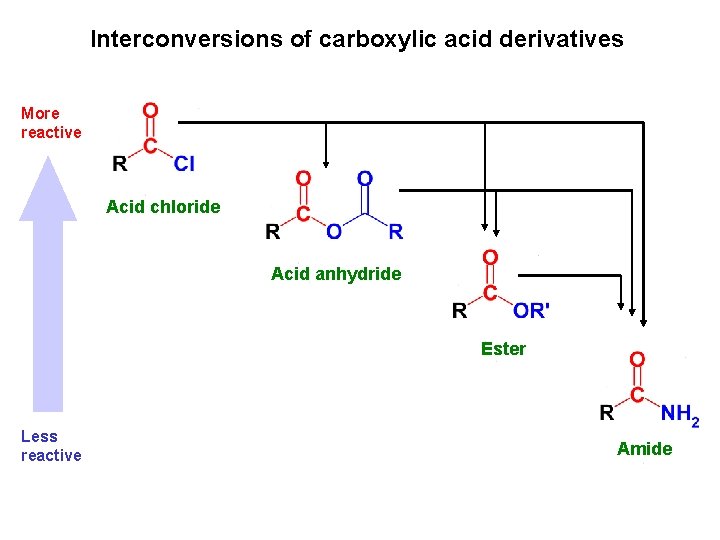
Interconversions of carboxylic acid derivatives More reactive Acid chloride Acid anhydride Ester Less reactive Amide
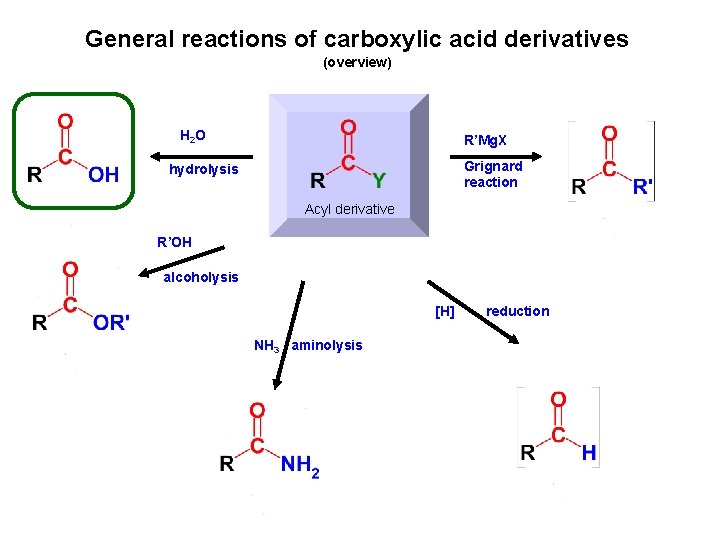
General reactions of carboxylic acid derivatives (overview) H 2 O R’Mg. X Grignard reaction hydrolysis Acyl derivative R’OH alcoholysis [H] NH 3 aminolysis reduction
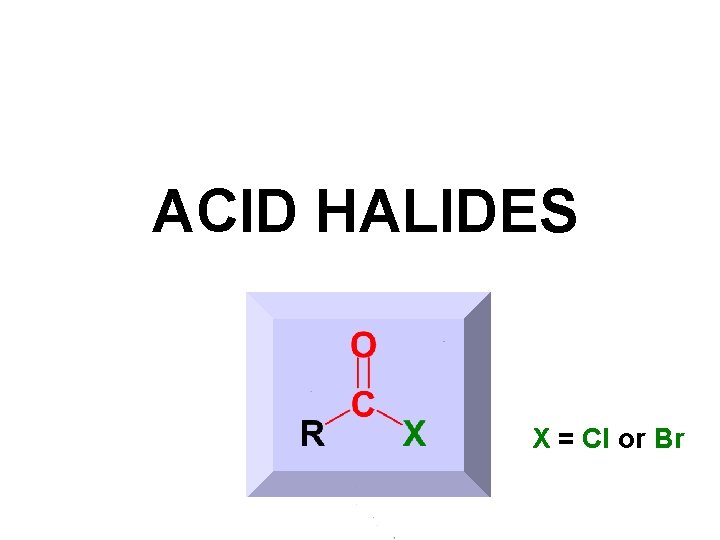
ACID HALIDES X = Cl or Br
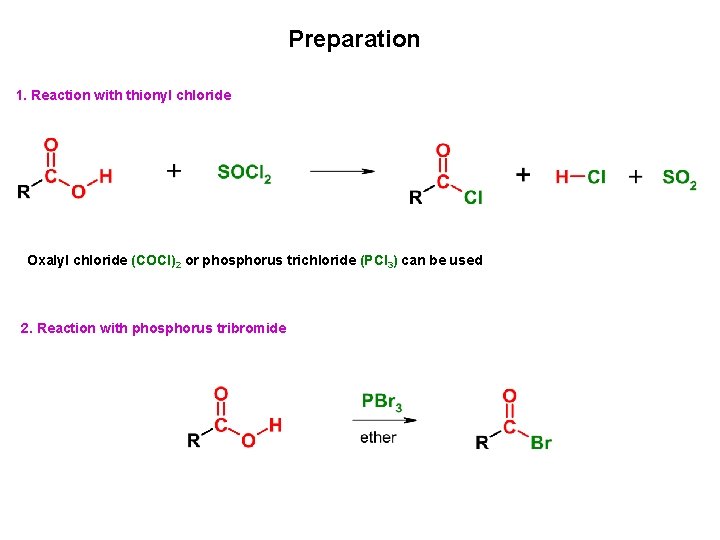
Preparation 1. Reaction with thionyl chloride Oxalyl chloride (COCl)2 or phosphorus trichloride (PCl 3) can be used 2. Reaction with phosphorus tribromide
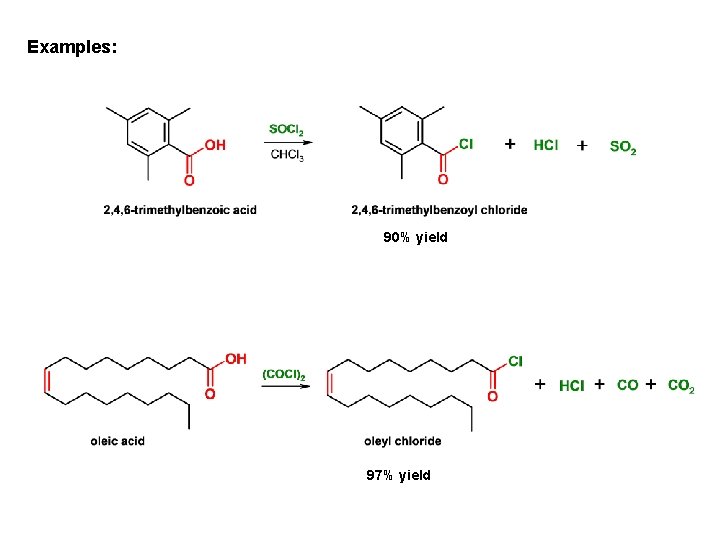
Examples: 90% yield 97% yield
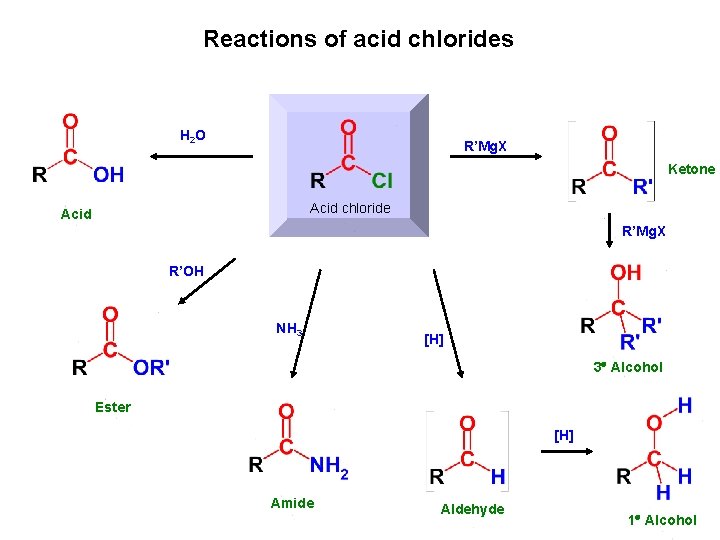
Reactions of acid chlorides H 2 O R’Mg. X Ketone Acid chloride Acid R’Mg. X R’OH NH 3 [H] 3 Alcohol Ester [H] Amide Aldehyde 1 Alcohol

Hydrolysis: conversion of acid chlorides into acids Using of acid chlorides as acylating agents requires anhydrous conditions

Alcoholysis: conversion of acid chlorides into esters Excellent method for esters preparation Amine (pyridine) added for HCl scavenging
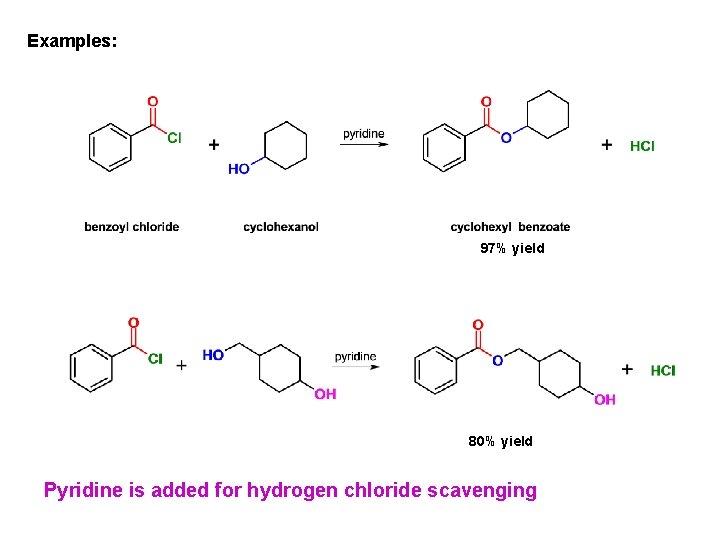
Examples: 97% yield 80% yield Pyridine is added for hydrogen chloride scavenging
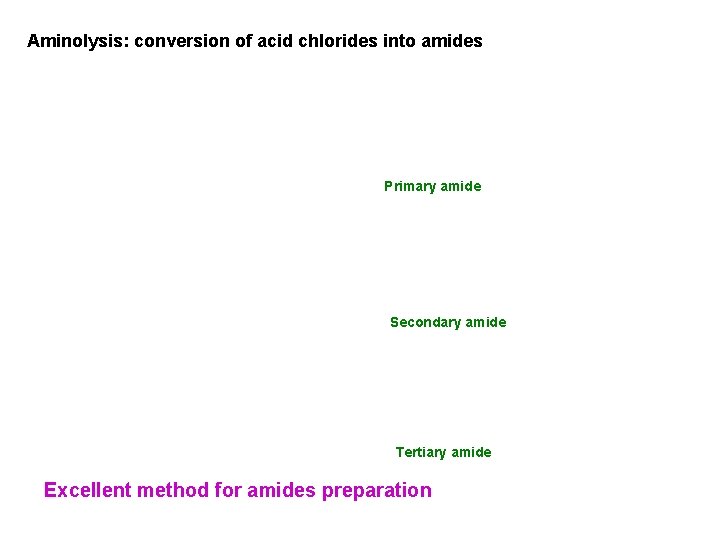
Aminolysis: conversion of acid chlorides into amides Primary amide Secondary amide Tertiary amide Excellent method for amides preparation

Example: Schotten-Baumann reaction Sedative agent (an amide)
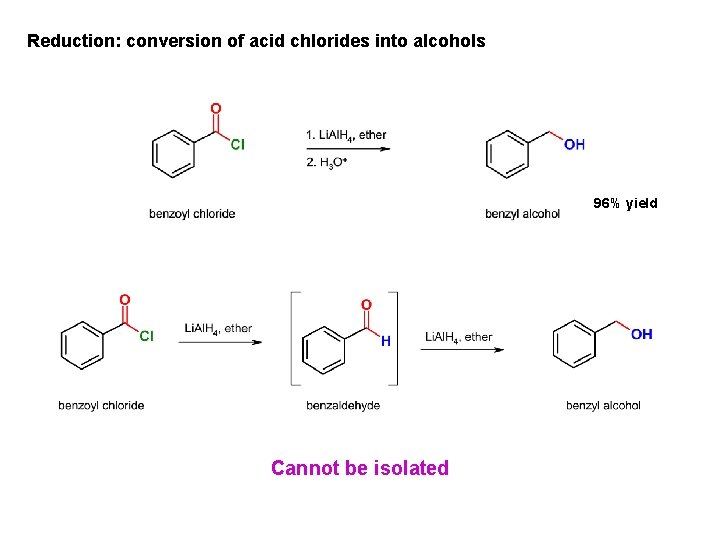
Reduction: conversion of acid chlorides into alcohols 96% yield Cannot be isolated
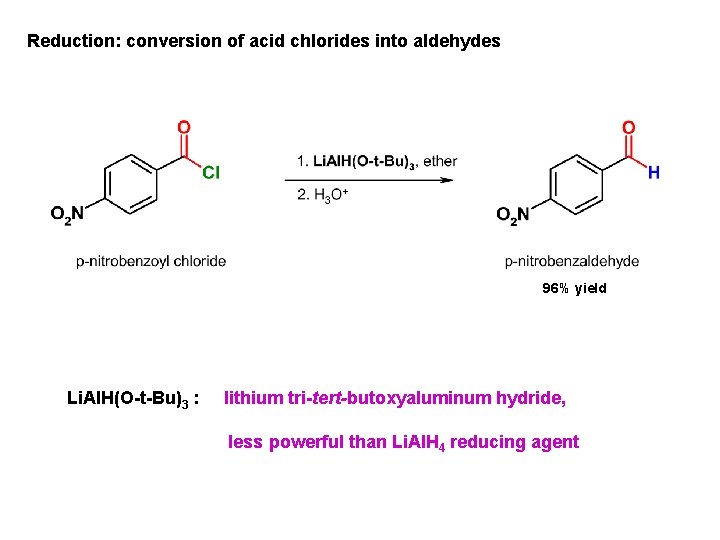
Reduction: conversion of acid chlorides into aldehydes 96% yield Li. Al. H(O-t-Bu)3 : lithium tri-tert-butoxyaluminum hydride, less powerful than Li. Al. H 4 reducing agent
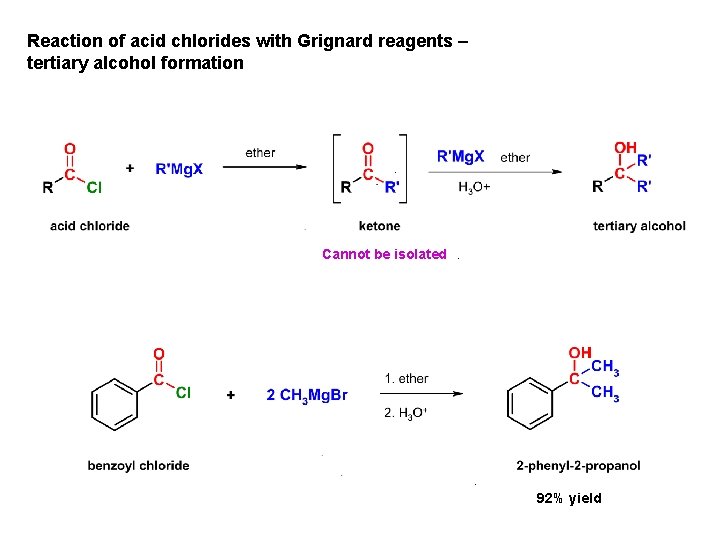
Reaction of acid chlorides with Grignard reagents – tertiary alcohol formation Cannot be isolated 92% yield
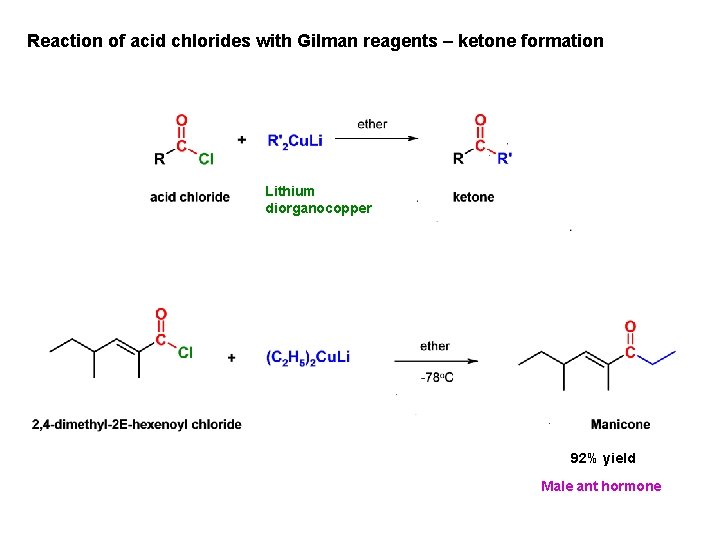
Reaction of acid chlorides with Gilman reagents – ketone formation Lithium diorganocopper 92% yield Male ant hormone
ACID ANHYDRIDES
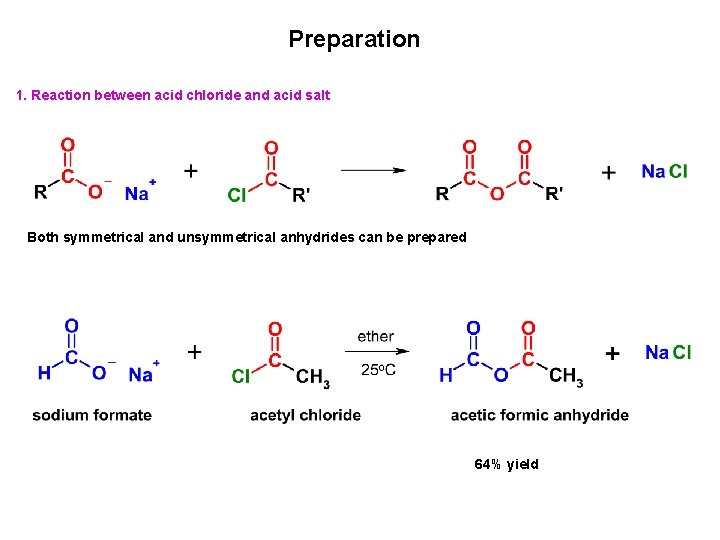
Preparation 1. Reaction between acid chloride and acid salt Both symmetrical and unsymmetrical anhydrides can be prepared 64% yield

Preparation 2. Dehydration of dicarboxylic acids – formation of cyclic anhydrides

2. Dehydration of dicarboxylic acids – formation of cyclic anhydrides
![Reactions of acid anhydrides [H] H 2 O Acid anhydride Aldehyde R’OH Acid Ester Reactions of acid anhydrides [H] H 2 O Acid anhydride Aldehyde R’OH Acid Ester](http://slidetodoc.com/presentation_image/5e684aa4e029ff3263b7e4e348e49965/image-27.jpg)
Reactions of acid anhydrides [H] H 2 O Acid anhydride Aldehyde R’OH Acid Ester NH 3 [H] Amide 1 Alcohol

Acetic anhydride is most frequently used for preparation of acetates of complex alcohols or substituted acetamides from amines. Examples: Component of anti headache drugs
ESTERS
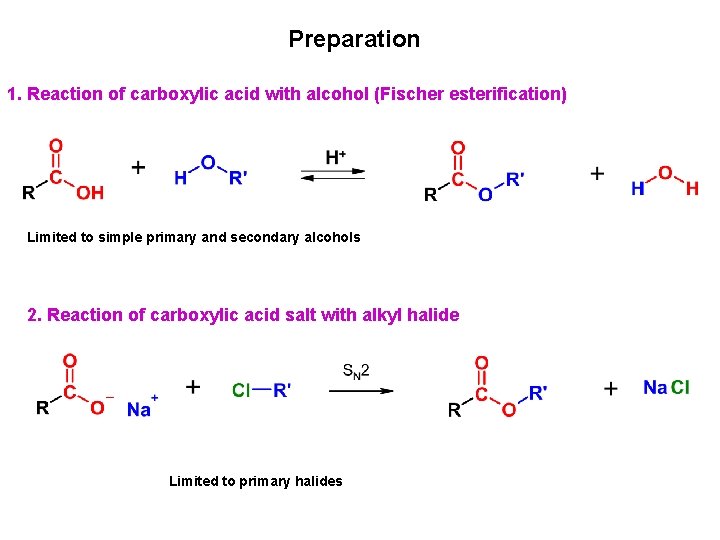
Preparation 1. Reaction of carboxylic acid with alcohol (Fischer esterification) Limited to simple primary and secondary alcohols 2. Reaction of carboxylic acid salt with alkyl halide Limited to primary halides
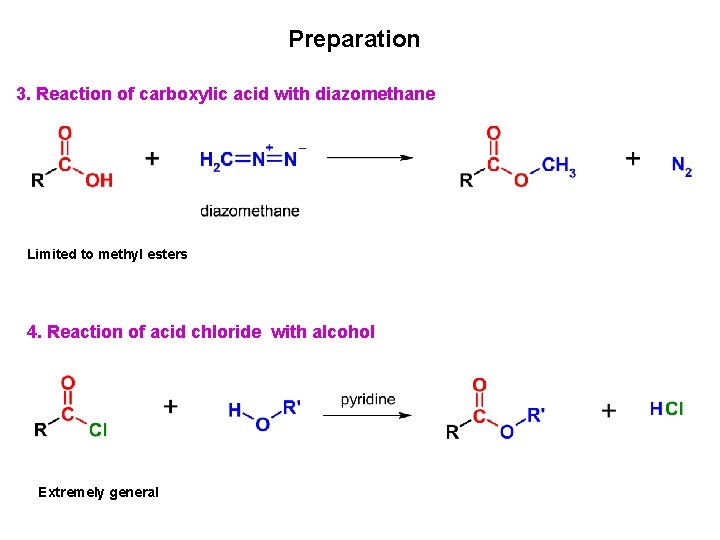
Preparation 3. Reaction of carboxylic acid with diazomethane Limited to methyl esters 4. Reaction of acid chloride with alcohol Extremely general
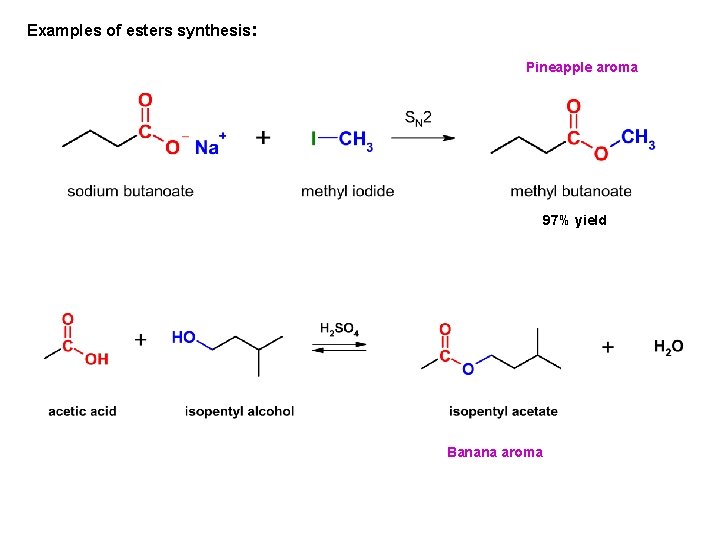
Examples of esters synthesis: Pineapple aroma 97% yield Banana aroma
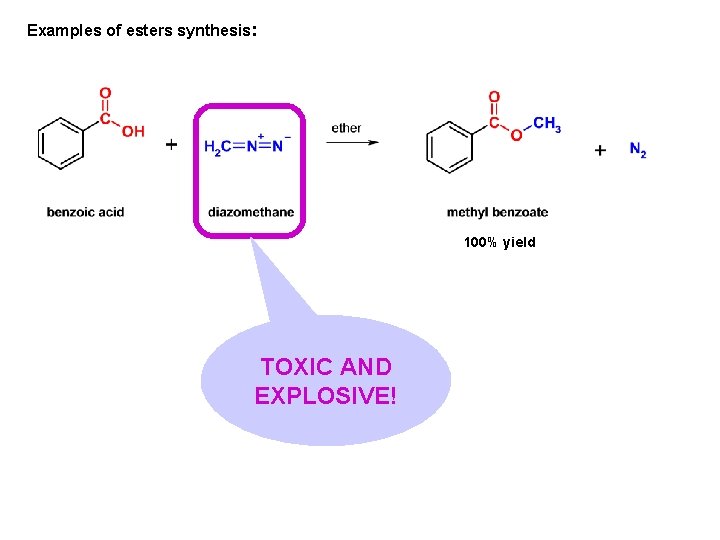
Examples of esters synthesis: 100% yield TOXIC AND EXPLOSIVE!

Esters in nature Plant oils and animal fats are esters of glycerol and carboxylic acids C 12 -C 22
![Reactions of esters [H] Ester H 2 O Aldehyde NH 3 Acid + 2 Reactions of esters [H] Ester H 2 O Aldehyde NH 3 Acid + 2](http://slidetodoc.com/presentation_image/5e684aa4e029ff3263b7e4e348e49965/image-35.jpg)
Reactions of esters [H] Ester H 2 O Aldehyde NH 3 Acid + 2 R’’Mg. X [H] Alcohol Amide 3 Alcohol 1 Alcohol

Acid catalysed hydrolysis of esters (reversible)

Base catalysed hydrolysis of esters (irreversible)
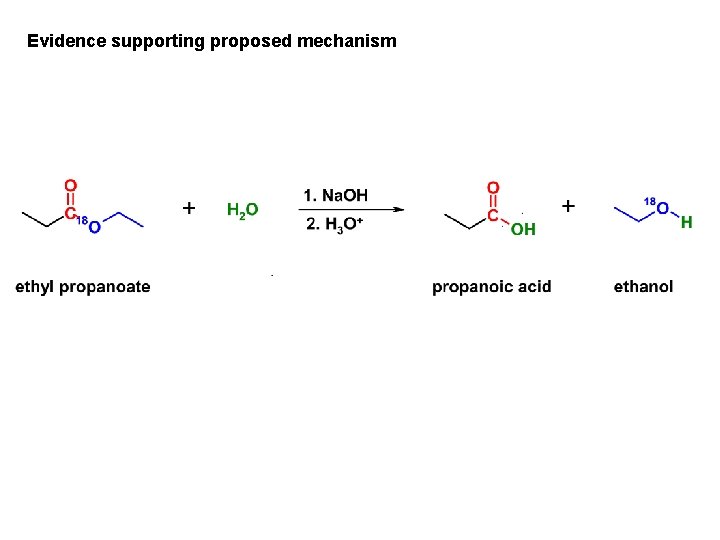
Evidence supporting proposed mechanism
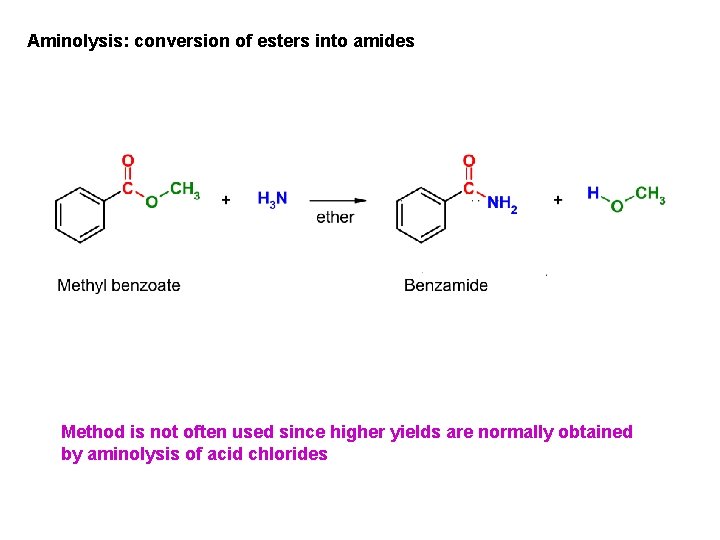
Aminolysis: conversion of esters into amides Method is not often used since higher yields are normally obtained by aminolysis of acid chlorides
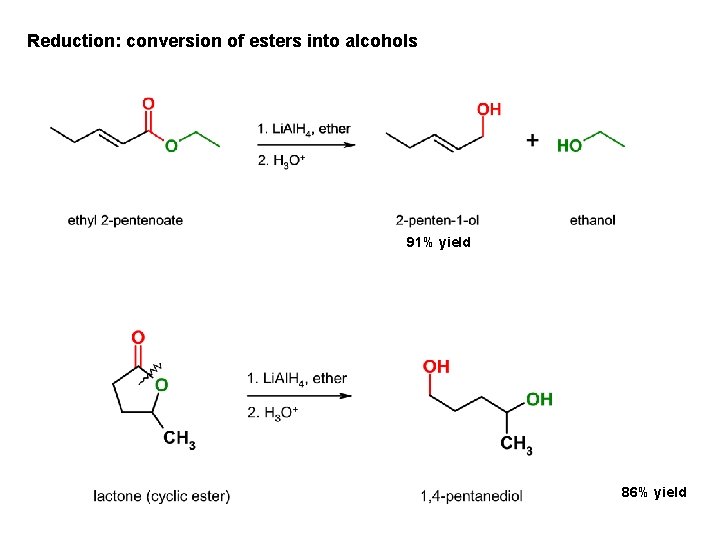
Reduction: conversion of esters into alcohols 91% yield 86% yield

Reduction: conversion of esters into aldehydes 91% yield DIBAH = DIISOBUTYLALUMINUM HYDRIDE (i-Bu)2 Al. H
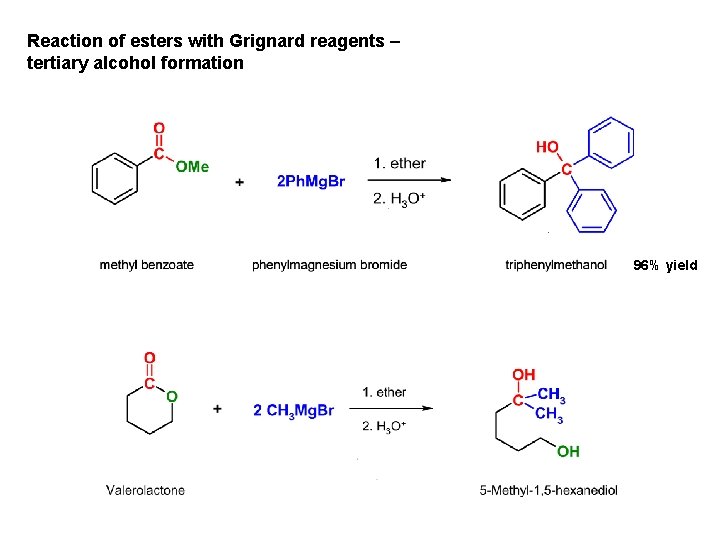
Reaction of esters with Grignard reagents – tertiary alcohol formation 96% yield
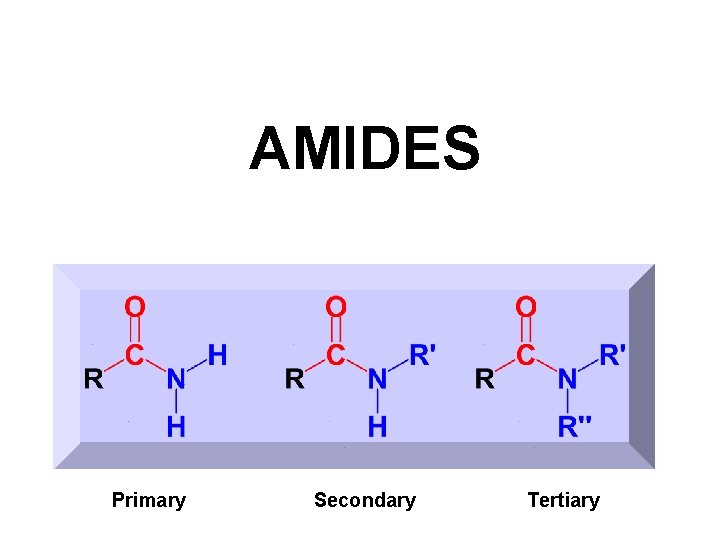
AMIDES Primary Secondary Tertiary

Preparation Primary amide Secondary amide Tertiary amide
Amides in nature Amide bonds
![Reactions of amides Acidic hydrolysis H 2 O, HCl Amide [H] Basic hydrolysis H Reactions of amides Acidic hydrolysis H 2 O, HCl Amide [H] Basic hydrolysis H](http://slidetodoc.com/presentation_image/5e684aa4e029ff3263b7e4e348e49965/image-46.jpg)
Reactions of amides Acidic hydrolysis H 2 O, HCl Amide [H] Basic hydrolysis H 2 O, Na. OH Amine
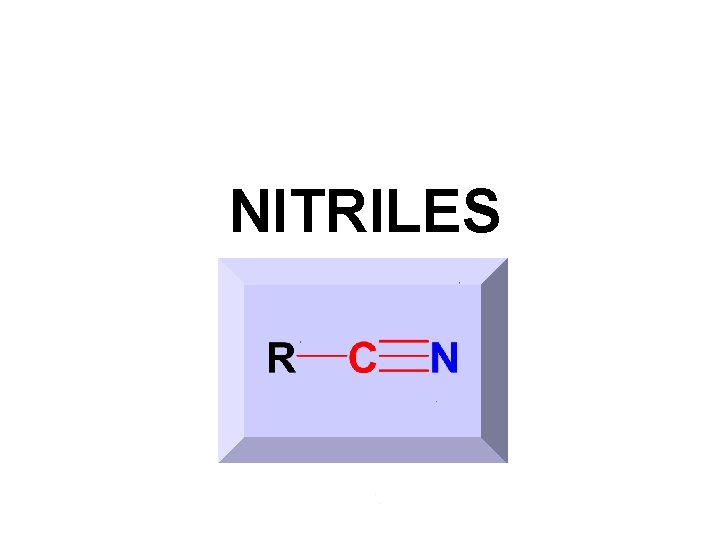
NITRILES
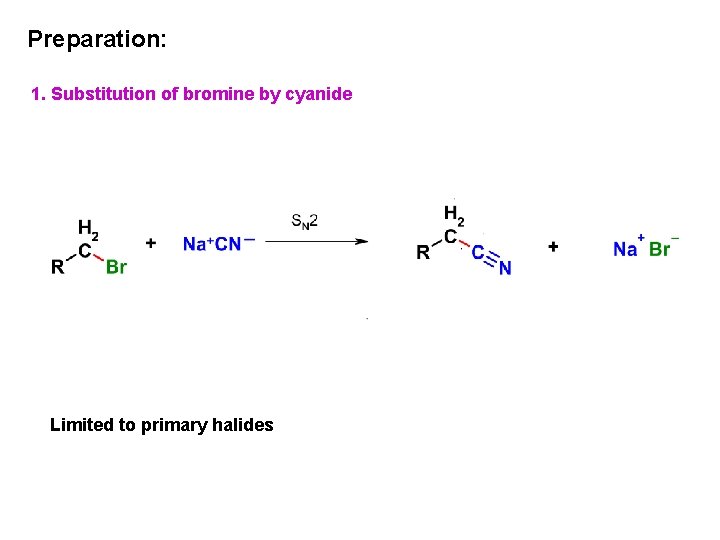
Preparation: 1. Substitution of bromine by cyanide Limited to primary halides
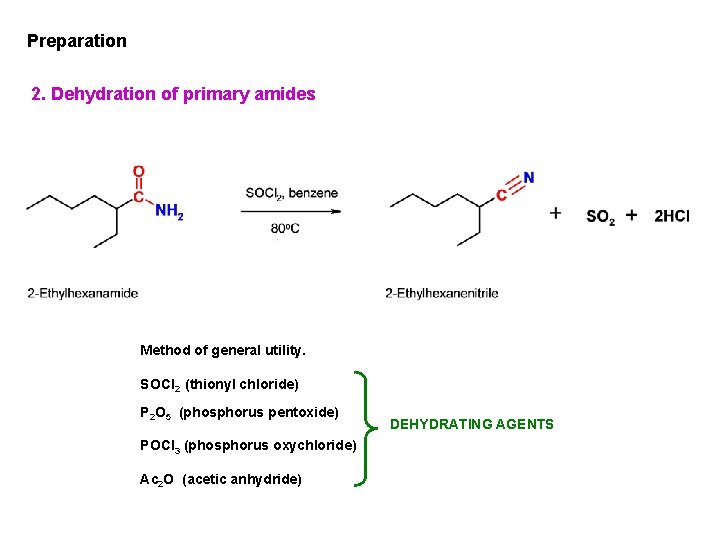
Preparation 2. Dehydration of primary amides Method of general utility. SOCl 2 (thionyl chloride) P 2 O 5 (phosphorus pentoxide) POCl 3 (phosphorus oxychloride) Ac 2 O (acetic anhydride) DEHYDRATING AGENTS

Similarity of nitriles to carbonyl compounds
![Reactions of nitriles Amide R’Mg. X Nitrile H 2 O [H] Ketone H 2 Reactions of nitriles Amide R’Mg. X Nitrile H 2 O [H] Ketone H 2](http://slidetodoc.com/presentation_image/5e684aa4e029ff3263b7e4e348e49965/image-51.jpg)
Reactions of nitriles Amide R’Mg. X Nitrile H 2 O [H] Ketone H 2 O Acid Amine Aldehyde
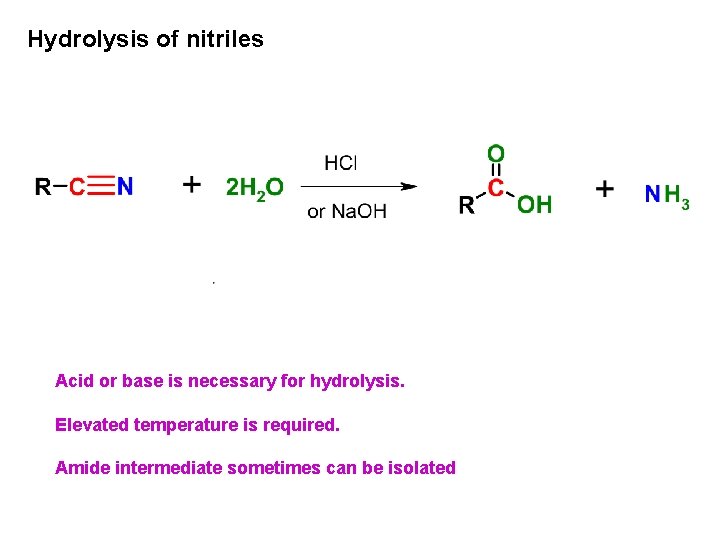
Hydrolysis of nitriles Acid or base is necessary for hydrolysis. Elevated temperature is required. Amide intermediate sometimes can be isolated

Reduction of nitriles
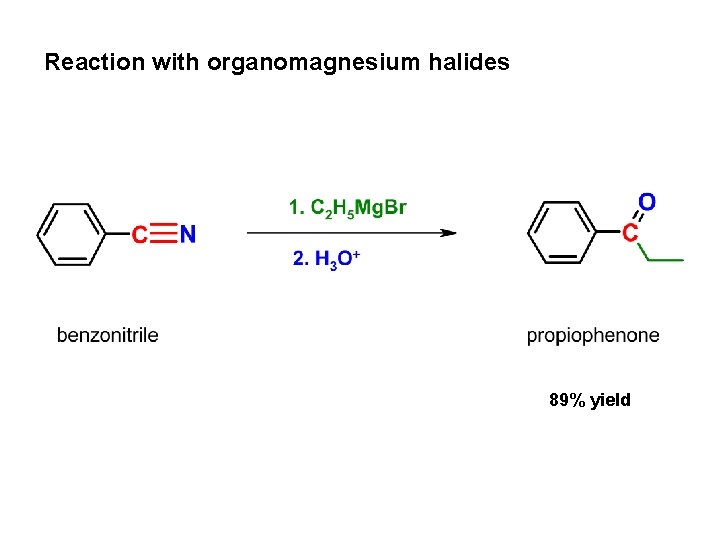
Reaction with organomagnesium halides 89% yield
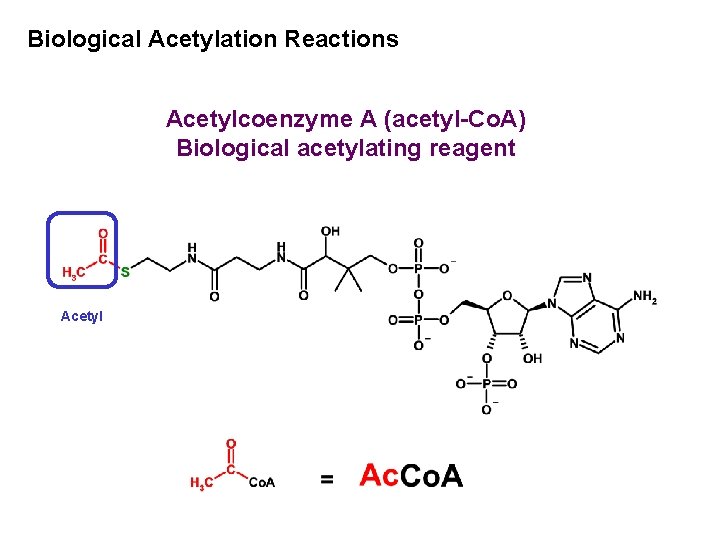
Biological Acetylation Reactions Acetylcoenzyme A (acetyl-Co. A) Biological acetylating reagent Acetyl

Acetylation of glucosylamine

α-Substitution of Carboxylic Acid Derivatives
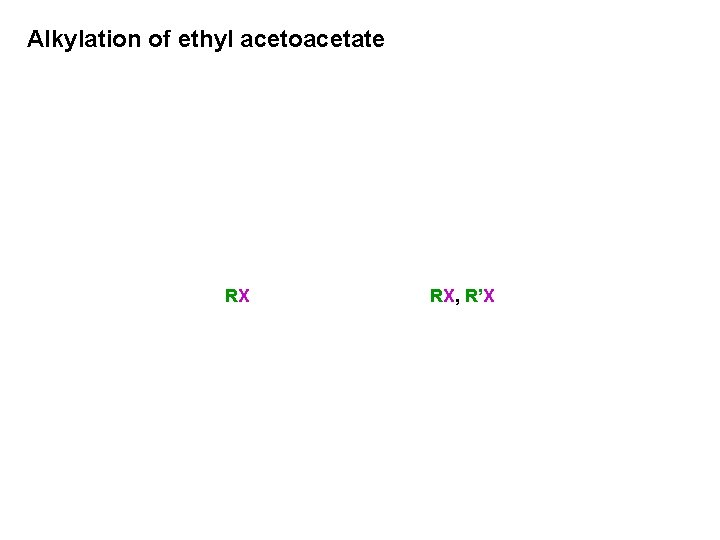
Alkylation of ethyl acetoacetate RX RX, R’X

Monoalkylation of ethyl acetoacetate

Dialkylation of ethyl acetoacetate
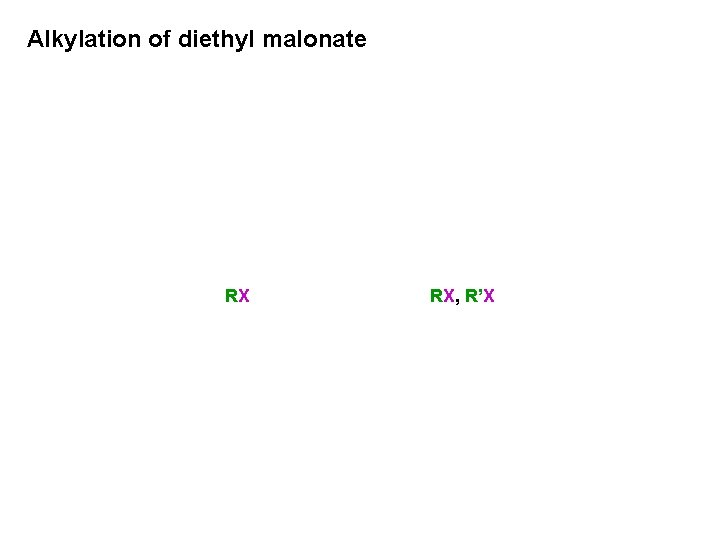
Alkylation of diethyl malonate RX RX, R’X

Alkylation of diethyl malonate

Condensations of Carboxylic Acid Derivatives

Claisen condensation of ethyl acetate 75% yield

Mixed Claisen condensation of esters (β-ketoester)

Mixed Claisen condensation 91% yield
Intramolecular Claisen condensation

Perkin condensation

Biological Condensation Reactions
Biological Condensation Reactions: Fatty acids synthesis (lipogenesis)
- Slides: 70