Carbonyl Compounds Carbonyl compounds C O Carbonyl group

Carbonyl Compounds

Carbonyl compounds C O Carbonyl group sp 2 hybridized carbon Coplanar bonds, 120 o bond angle p-p overlap bond Two types of compounds H Aldehyde C R O R’ Ketone C R O
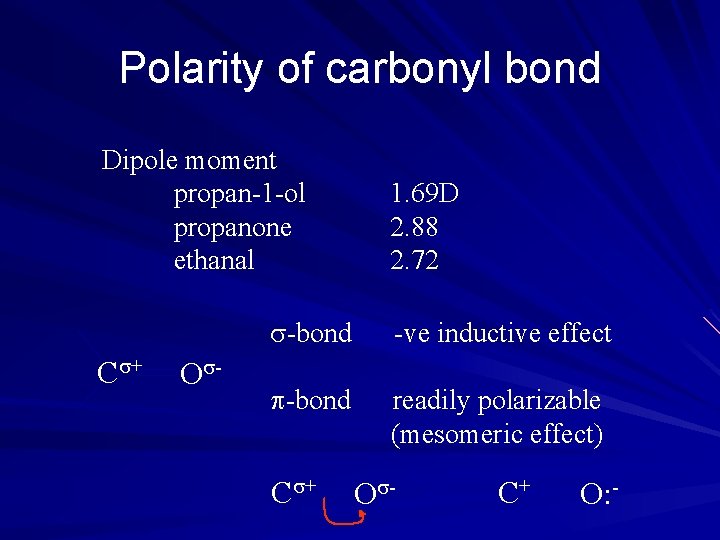
Polarity of carbonyl bond Dipole moment propan-1 -ol propanone ethanal C + O - 1. 69 D 2. 88 2. 72 -bond -ve inductive effect -bond readily polarizable (mesomeric effect) C + O - C+ O: -
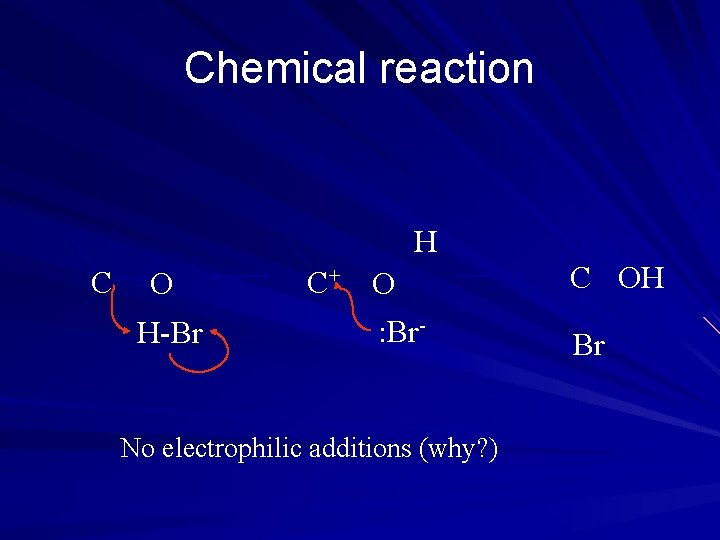
Chemical reaction H C O H-Br C+ O : Br- No electrophilic additions (why? ) C OH Br
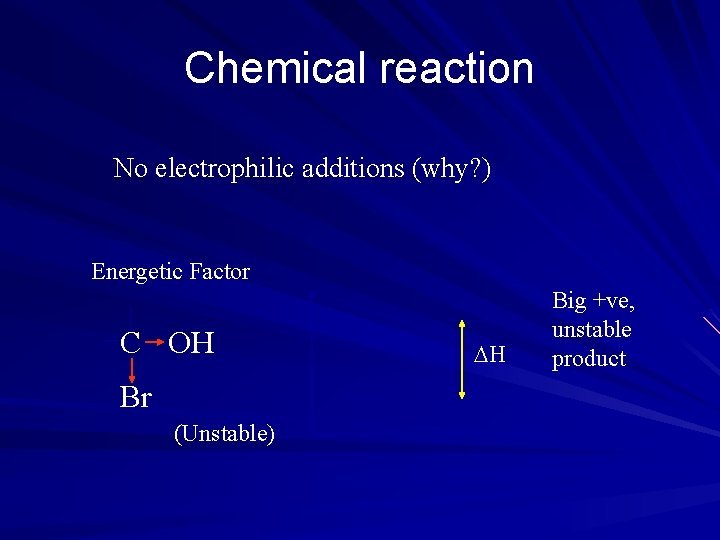
Chemical reaction No electrophilic additions (why? ) Energetic Factor C OH Br (Unstable) H Big +ve, unstable product

Chemical reaction No electrophilic additions (why? ) Big +ve Ea , unstable Transition state Kinetic Factor C+ OH (Unstable) Ea
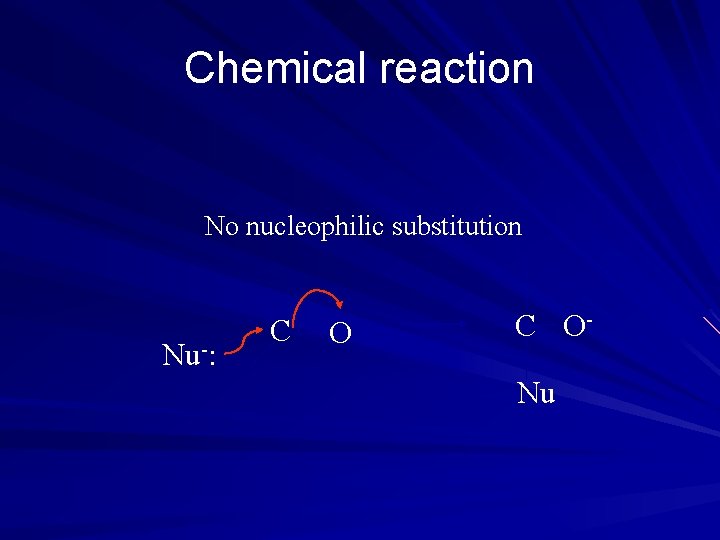
Chemical reaction No nucleophilic substitution Nu-: C ONu
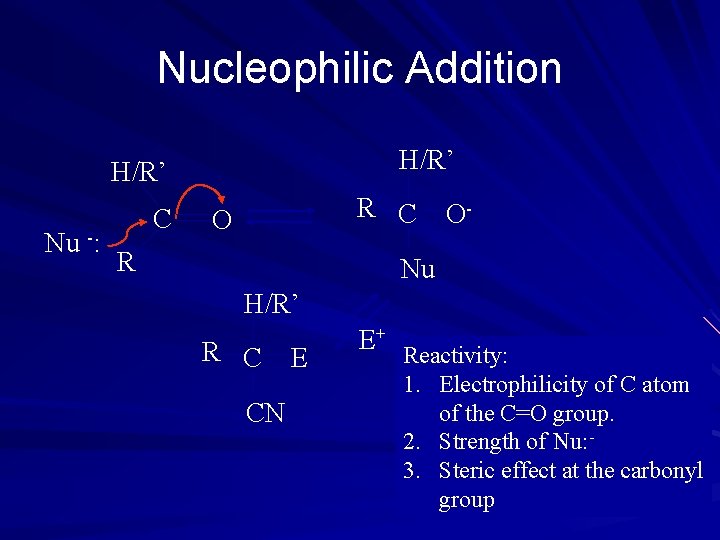
Nucleophilic Addition H/R’ Nu -: C R C O R O- Nu H/R’ R C CN E E+ Reactivity: 1. Electrophilicity of C atom of the C=O group. 2. Strength of Nu: 3. Steric effect at the carbonyl group
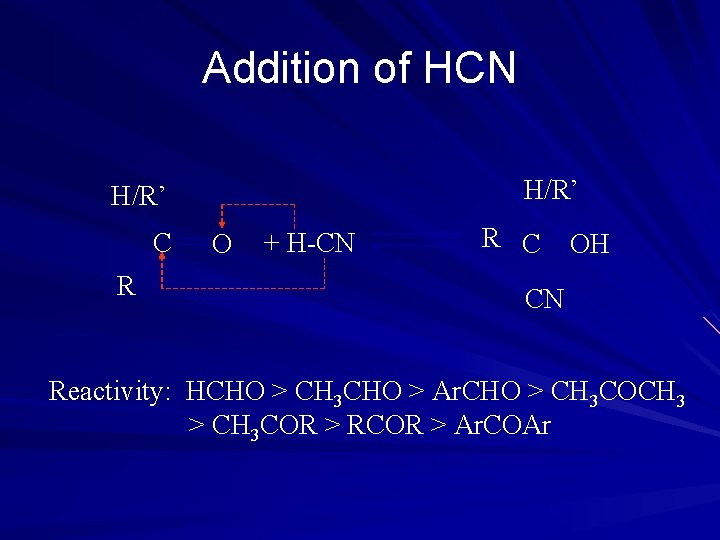
Addition of HCN H/R’ C R O + H-CN R C OH CN Reactivity: HCHO > CH 3 CHO > Ar. CHO > CH 3 COCH 3 > CH 3 COR > RCOR > Ar. COAr
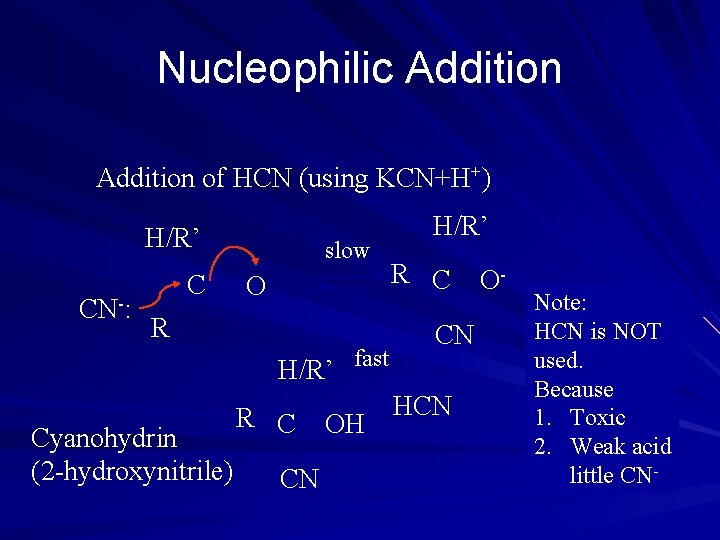
Nucleophilic Addition of HCN (using KCN+H+) H/R’ CN-: C slow O R H/R’ fast Cyanohydrin (2 -hydroxynitrile) R C CN OH H/R’ R C CN HCN O- Note: HCN is NOT used. Because 1. Toxic 2. Weak acid little CN-
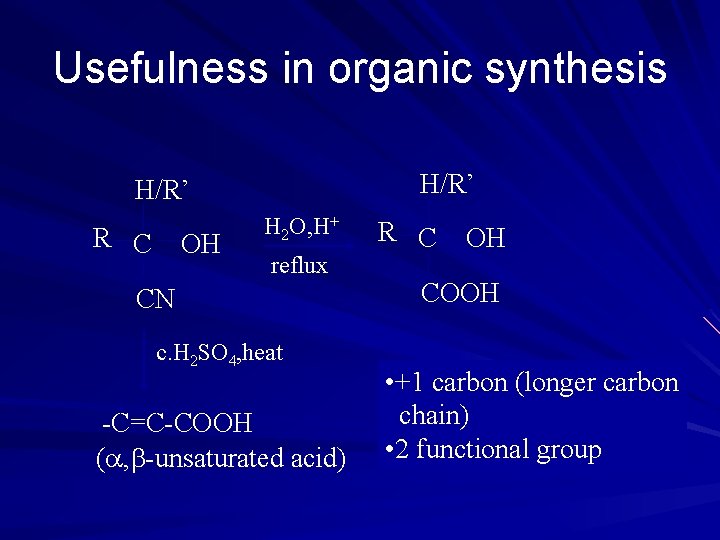
Usefulness in organic synthesis H/R’ R C OH H 2 O, H+ reflux CN c. H 2 SO 4, heat -C=C-COOH ( , -unsaturated acid) R C OH COOH • +1 carbon (longer carbon chain) • 2 functional group
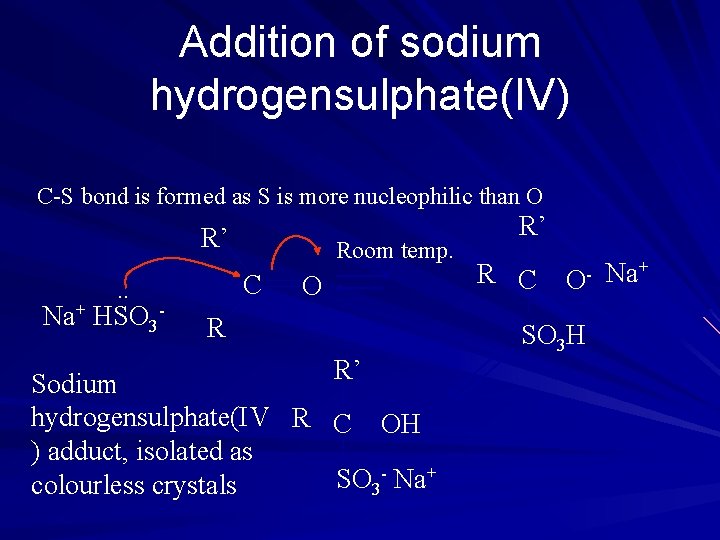
Addition of sodium hydrogensulphate(IV) C-S bond is formed as S is more nucleophilic than O R’ C . . Na+ HSO 3 - Room temp. O R R’ Sodium hydrogensulphate(IV R C OH ) adduct, isolated as - Na+ SO colourless crystals 3 R’ R C O- SO 3 H Na+
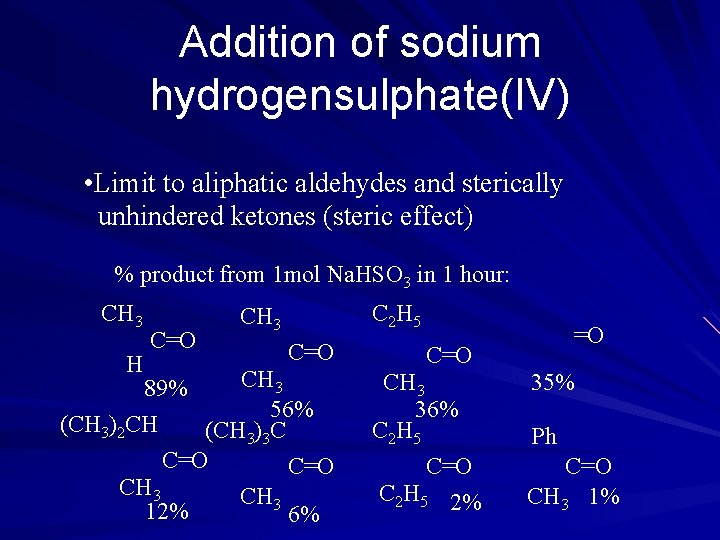
Addition of sodium hydrogensulphate(IV) • Limit to aliphatic aldehydes and sterically unhindered ketones (steric effect) % product from 1 mol Na. HSO 3 in 1 hour: CH 3 H C=O C 2 H 5 CH 3 C=O CH 3 89% 56% (CH 3)2 CH (CH 3)3 C C=O CH 3 12% 6% =O CH 3 36% C 2 H 5 C=O C 2 H 5 2% 35% Ph C=O CH 3 1%

Addition of sodium hydrogensulphate(IV) • Reversible (can be reversed by aq. Alkali or acid by shifting eqm. position to LHS by HSO 3 - + H+=> SO 2 , HSO 3 - + OH- => SO 32 -) • Use to purify liquid or gaseous carbonyl compounds which are difficult to purify by direct recrystallization.
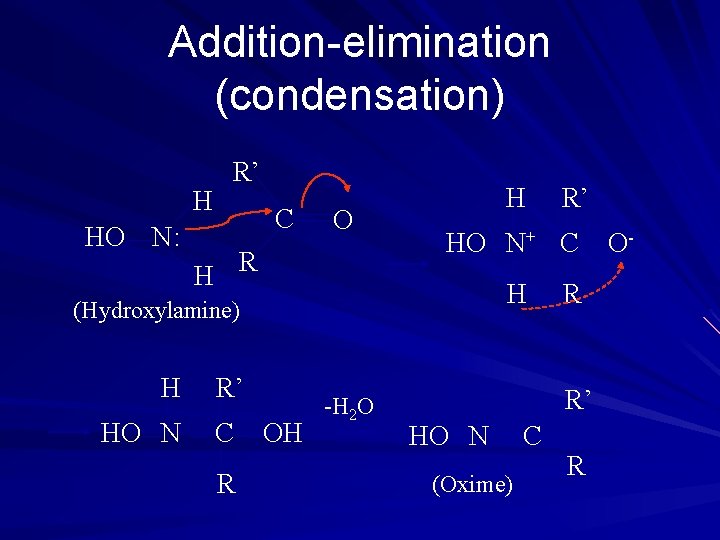
Addition-elimination (condensation) R’ H C HO N: O R H H HO N+ C H (Hydroxylamine) H HO N R’ C R OH -H 2 O R’ R R’ HO N (Oxime) C R O-
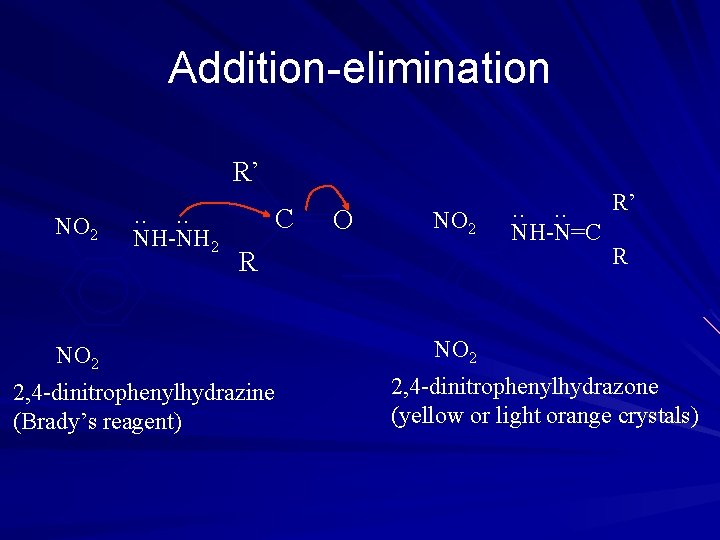
Addition-elimination R’ NO 2 . . NH-NH 2 C O NO 2 R NO 2 2, 4 -dinitrophenylhydrazine (Brady’s reagent) . . NH-N=C R’ R NO 2 2, 4 -dinitrophenylhydrazone (yellow or light orange crystals)

Phenylhydrazone • Products have sharp and characteristic melting point. • Used as the identification of the original aldehyde and ketone Note: 1. NH 3 does not react 2. Predict the product obtained by adding H 2 N-NH 2 to propanal.

Oxidation 1. KMn. O 4/H+ , K 2 Cr 2 O 7/H+ (Strong oxidizing agent) RCHO => RCOOH RCH 2 COCH 2 R’ => RCOOH + R’ CH 2 COOH + R’COOH C 6 H 5 CHO => C 6 H 5 COOH requiring reflux for hours

Oxidation 2. Tollen’s reagent (silver mirror test) Reagent: 2 Ag+ + 2 OH- => Ag 2 O + H 2 O Ag 2 O + 4 NH 3 + H 2 O => 2 Ag(NH 3)2 OH 2[Ag(NH 3)2]+ + RCHO + 3 OH=> RCOO- +2 H 2 O + 4 NH 3 + 2 Ag (mirror) No reaction with ketone (Tollen’s reagent is a mild O. A. )

Oxidation 3. Fehling’s reagent Reagent: alkaline solution of copper(II) tartrate RCHO + 2 Cu 2+ + 5 OH- => RCOO- + 3 H 2 O + Cu 2 O (Fehling) (brick-red) Note: No reaction with Ketones and Aromatic Aldehydes

Reduction Reducing agent: Li. Al. H 4 Lithium Tetrahydridoaluminate Na. BH 4 Sodium Tetrahydridoborate Both equivalent to a source of hydride ion, H-. R R C O H/R H- C H/R OH H+ R OH C H/R H

Reduction Li. Al. H 4 must be kept dry i. e. in solution of dry ether Li. BH 4 is less powerful, can be used in aqueous solution. Reducing agent: H 2/Ni, similar to alkene R C H/R O H 2/Ni RCH 2 OH
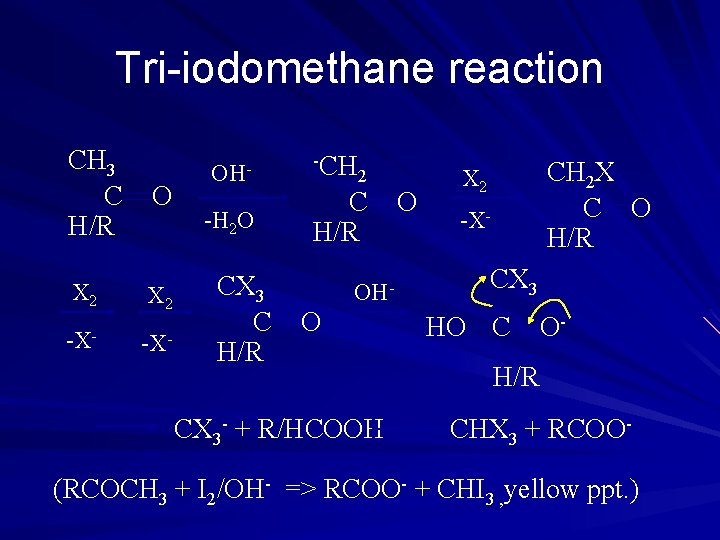
Tri-iodomethane reaction CH 3 C H/R X 2 -X- OH-H 2 O CX 3 C H/R -CH 2 C H/R OH- O CX 3 - + R/HCOOH O CH 2 X C O H/R X 2 -X- CX 3 HO C O- H/R CHX 3 + RCOO- (RCOCH 3 + I 2/OH- => RCOO- + CHI 3 , yellow ppt. )
- Slides: 23