Carbon monoxide CO The natural sources in atmosphere


Carbon monoxide ( CO ) The natural sources in atmosphere are • volcanic activity. • most natural CO gas are from marshes (CH 4 emission) depending upon the (methane oxidation) hʋ O 3 O 2 + O. + H 2 O OH. + OH. CH 4 + OH. H 2 O + CH 3. CH 4 + O. OH. + CH 3. Net results --------------------- CH 3. + 2 O 2 CO + 3 OH.

Man-made (human activities) sourses (1) Automobile exhausts (which accounts for 60% of CO in the atmosphere), Since transportation sector accounts for about 74% of the entire global CO-emissions, (2) Forest fires and agricultural burning (3) Industrial operations such as electric and blast furnaces in iron & steel industry, petroleum refining, paper industry, and coal mining.
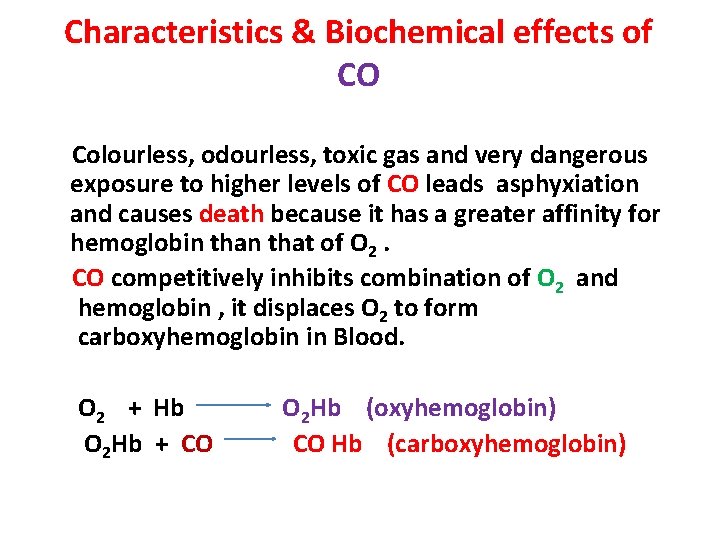
Characteristics & Biochemical effects of CO Colourless, odourless, toxic gas and very dangerous exposure to higher levels of CO leads asphyxiation and causes death because it has a greater affinity for hemoglobin that of O 2. CO competitively inhibits combination of O 2 and hemoglobin , it displaces O 2 to form carboxyhemoglobin in Blood. O 2 + Hb O 2 Hb (oxyhemoglobin) O 2 Hb + CO Hb (carboxyhemoglobin)
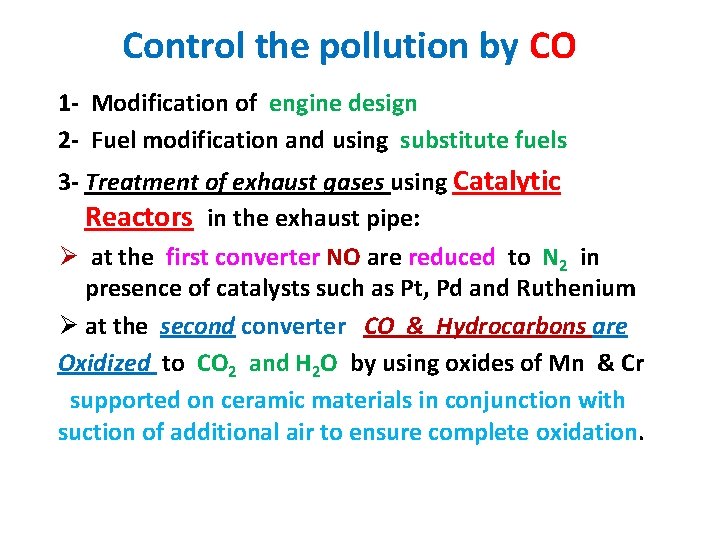
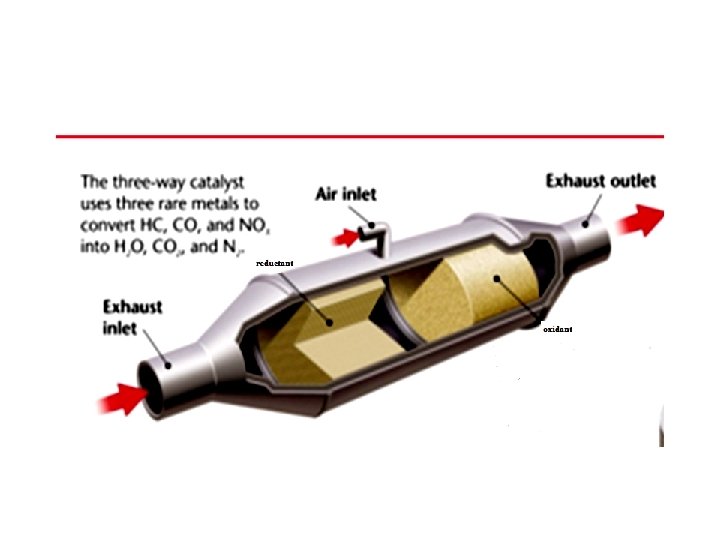
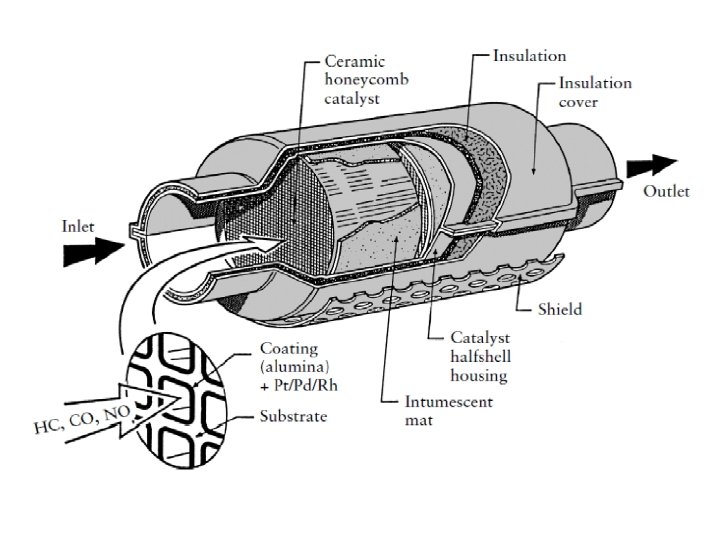
Control the pollution by CO 1 - Modification of engine design 2 - Fuel modification and using substitute fuels 3 - Treatment of exhaust gases using Catalytic Reactors in the exhaust pipe: Ø at the first converter NO are reduced to N 2 in presence of catalysts such as Pt, Pd and Ruthenium Ø at the second converter CO & Hydrocarbons are Oxidized to CO 2 and H 2 O by using oxides of Mn & Cr supported on ceramic materials in conjunction with suction of additional air to ensure complete oxidation.
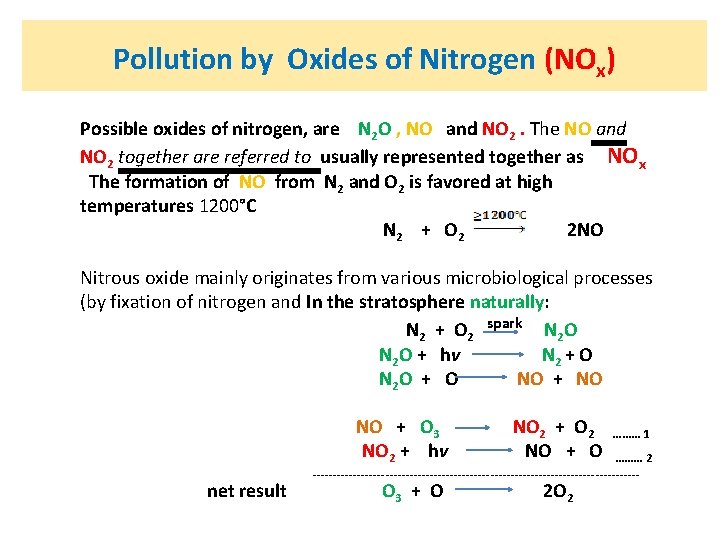
Pollution by Oxides of Nitrogen (NOx) Possible oxides of nitrogen, are N 2 O , NO and NO 2. The NO and NO 2 together are referred to usually represented together as NOx The formation of NO from N 2 and O 2 is favored at high temperatures 1200°C N 2 + O 2 2 NO Nitrous oxide mainly originates from various microbiological processes (by fixation of nitrogen and In the stratosphere naturally: N 2 + O 2 spark N 2 O N 2 O + hv N 2 + O N 2 O + O NO + NO NO + O 3 NO 2 + O 2 ……… 1 NO 2 + hv NO + O ……… 2 ----------------------------------------- net result O 3 + O 2 O 2
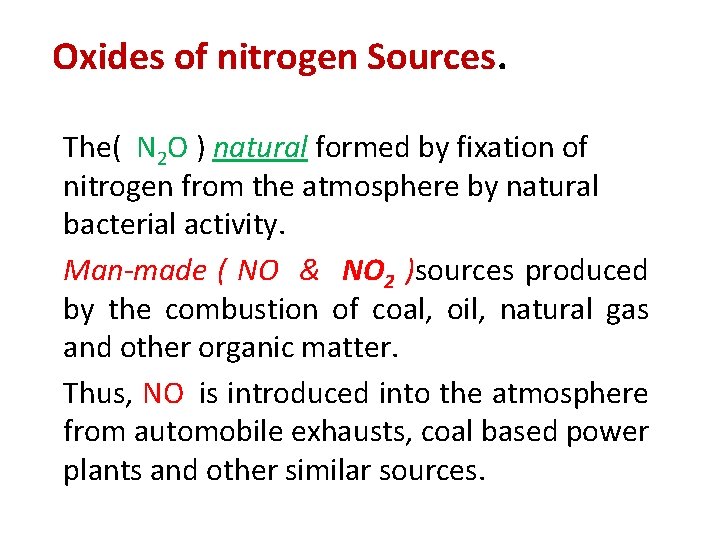
Oxides of nitrogen Sources. The( N 2 O ) natural formed by fixation of nitrogen from the atmosphere by natural bacterial activity. Man-made ( NO & NO 2 )sources produced by the combustion of coal, oil, natural gas and other organic matter. Thus, NO is introduced into the atmosphere from automobile exhausts, coal based power plants and other similar sources.
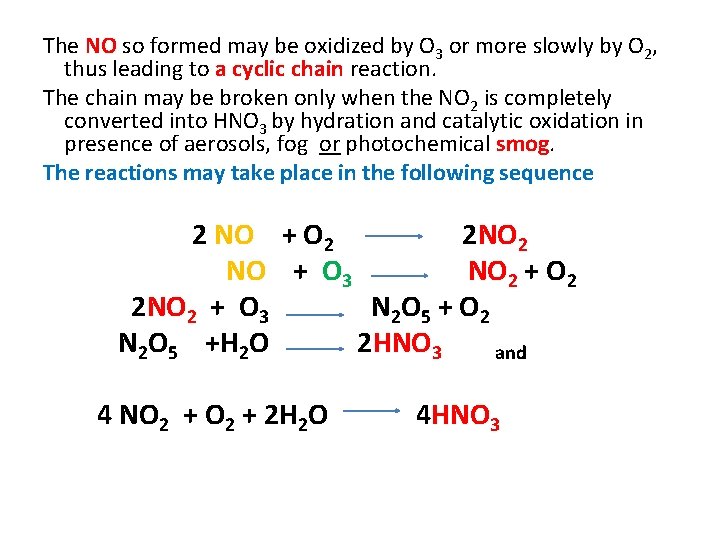
The NO so formed may be oxidized by O 3 or more slowly by O 2, thus leading to a cyclic chain reaction. The chain may be broken only when the NO 2 is completely converted into HNO 3 by hydration and catalytic oxidation in presence of aerosols, fog or photochemical smog. The reactions may take place in the following sequence 2 NO + O 2 2 NO 2 NO + O 3 NO 2 + O 2 2 NO 2 + O 3 N 2 O 5 + O 2 N 2 O 5 +H 2 O 2 HNO 3 and 4 NO 2 + 2 H 2 O 4 HNO 3
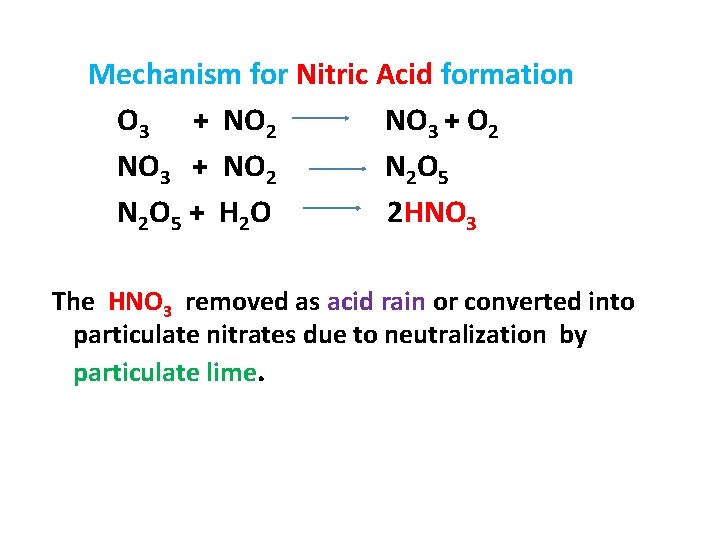
Mechanism for Nitric Acid formation O 3 + NO 2 NO 3 + O 2 NO 3 + NO 2 N 2 O 5 + H 2 O 2 HNO 3 The HNO 3 removed as acid rain or converted into particulate nitrates due to neutralization by particulate lime.
photo chemicalsmog Is a type of air pollution due to : Reaction of solar radiation with airborne pollutant mixtures of nitrogen oxides (Nox) This phenomenon takes place during sunny days with low winds and low level inversion. The photochemical smog and the consequent formation of aerosols reduce the visibility, cause irritation to eyes and damage plants and rubber goods.

Formation of photochemical Smog Step 1: People begin driving vehicles in the morning, nitrogen is burned or oxidized N 2 + O 2 → 2 NO Step 2: After a few hours, NO combines with O 2, in another oxidation reaction 2 NO + O 2 → 2 NO 2 Step 3: Nitrogen dioxide absorbs light energy, resulting in a reduction reaction NO 2 → NO + O Step 4: In sunlight, atomic oxygen combines with oxygen gas to form ozone O + O 2 → O 3 ( Bad ozone ) Step 5: Reaction is temperature and sunlight dependent O 3 + NO → NO 2 + O 2
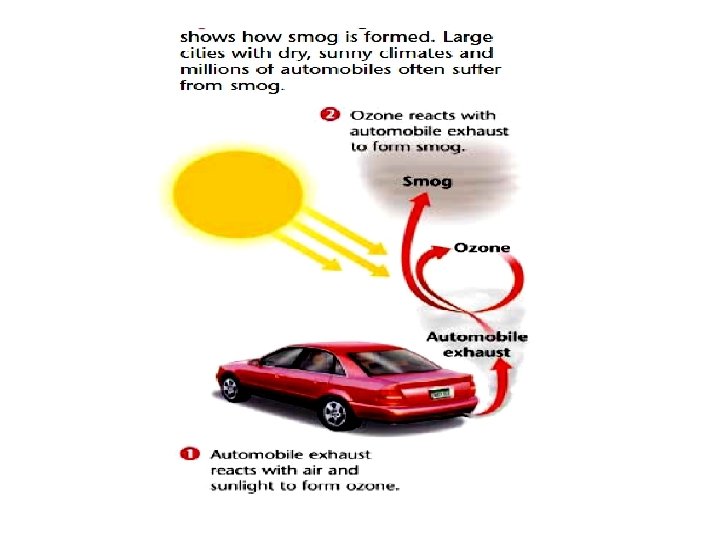

Bad Ozone is a pale blue gas, fairly water soluble, unstable, sweetish odour , Very reactive oxidizing agent if inhalation and absorption in the lungs causing accumulation of fluids in the lungs damaging lung capillaries.
PAN (peroxyacetyl nitrate) When the atmosphere is loaded with large quantities of automobile exhausts during warm sunny days, the exhaust gases are exposed to intense sunlight. A number of photochemical reactions involving NO , volatile organic compound and free radicals take place leading to the formation of PAN
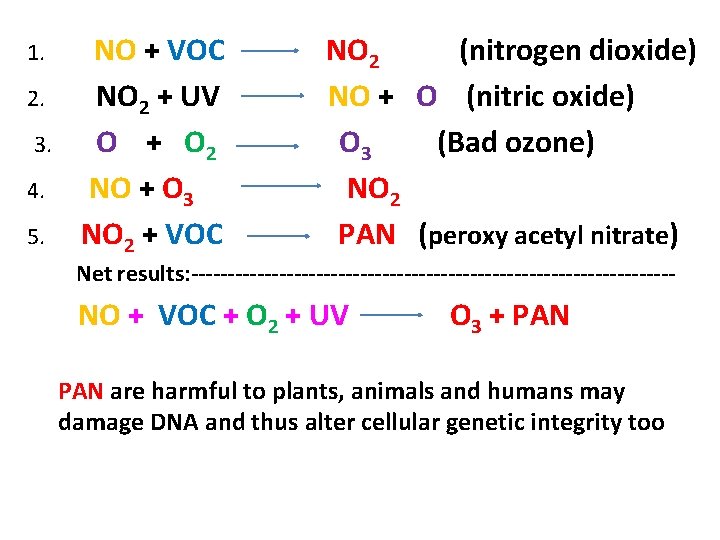
1. NO + VOC NO 2 (nitrogen dioxide) 2. NO 2 + UV NO + O (nitric oxide) 3. O + O 2 O 3 (Bad ozone) 4. NO + O 3 NO 2 5. NO 2 + VOC PAN (peroxy acetyl nitrate) Net results: --------------------------------- NO + VOC + O 2 + UV O 3 + PAN are harmful to plants, animals and humans may damage DNA and thus alter cellular genetic integrity too
Characteristics & Biochemical effects Ø NO 2 can travel into the respiratory system. Forms bonds with hemoglobin and reduces the efficiency of oxygen transport. ØDisrupts some cellular enzyme systems. Ø Higher levels and prolonged exposures may cause pulmonary fibrosis, inflammation of lung tissues and may eventually lead to death.
Control pollution by oxides of nitrogen NO from power plant emissions can be reduced to about 90% by using a two-stage combustion process. The fuel can be first fired at a high temperature using only about 90% of the stoichiometric air required so that only a minimum quantity of NO is formed under these conditions. Then the combustion of the fuel may be completed at a relatively low temperature, in the excess air. NO is not formed under these conditions.
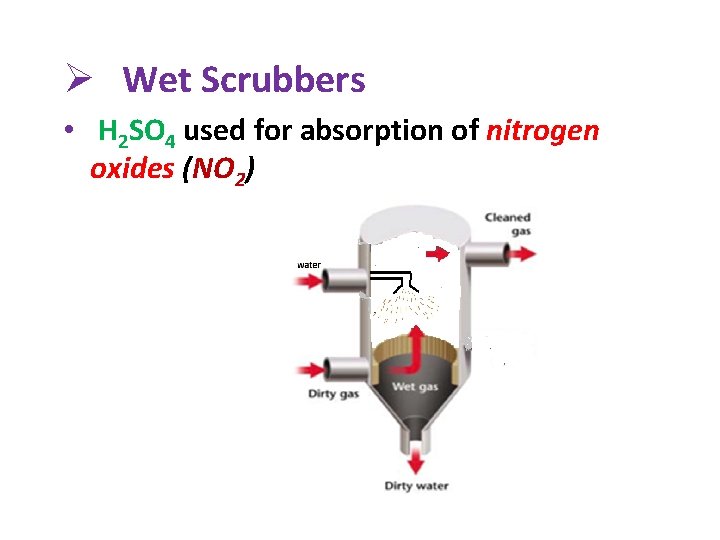
Ø Wet Scrubbers • H 2 SO 4 used for absorption of nitrogen oxides (NO 2)
Ø Reduction by CH 4 with Catalyst ( Pt ) Burning to convert (NO 2) to N 2 • CH 4 + NO 2 → NO +CO 2 +2 H 2 O • CH 4 + NO → CO 2 +2 H 2 O +N 2 • CH 4 +2 O 2 → CO 2 +2 H 2 O
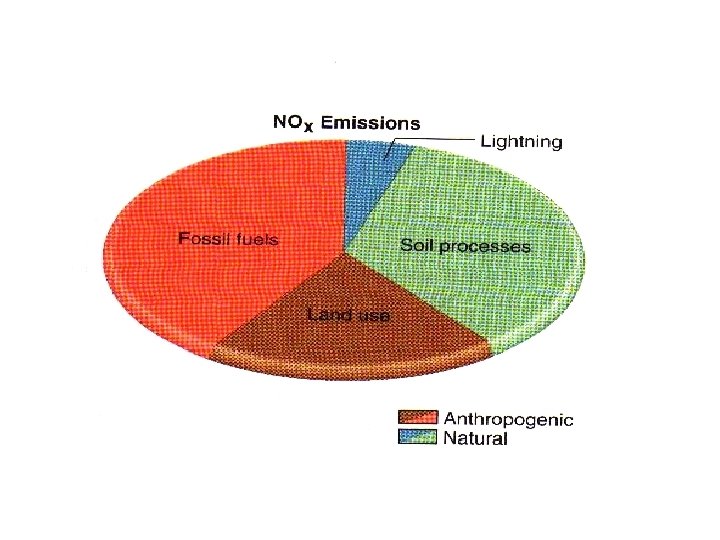
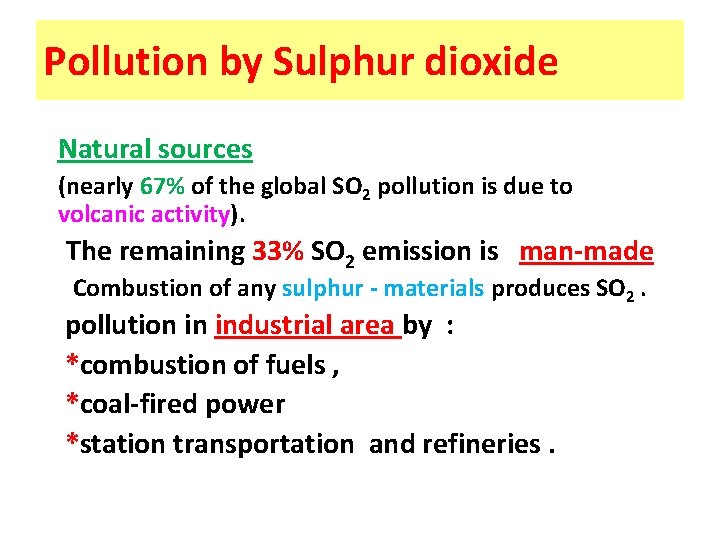
Pollution by Sulphur dioxide Natural sources (nearly 67% of the global SO 2 pollution is due to volcanic activity). The remaining 33% SO 2 emission is man-made Combustion of any sulphur - materials produces SO 2. pollution in industrial area by : *combustion of fuels , *coal-fired power *station transportation and refineries.
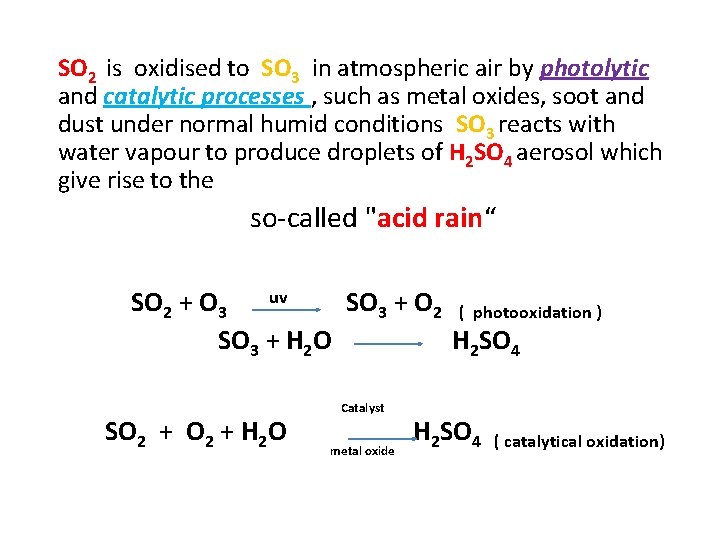
SO 2 is oxidised to SO 3 in atmospheric air by photolytic and catalytic processes , such as metal oxides, soot and dust under normal humid conditions SO 3 reacts with water vapour to produce droplets of H 2 SO 4 aerosol which give rise to the so-called "acid rain“ SO 2 + O 3 uv SO 3 + O 2 ( photooxidation ) SO 3 + H 2 O H 2 SO 4 Catalyst SO 2 + H 2 O H 2 SO 4 ( catalytical oxidation) metal oxide
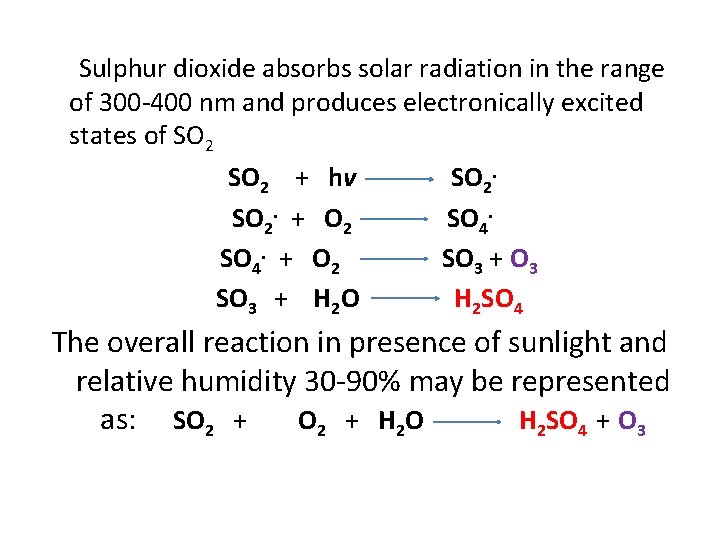
Sulphur dioxide absorbs solar radiation in the range of 300 -400 nm and produces electronically excited states of SO 2 + hv SO 2. + O 2 SO 4. + O 2 SO 3 + O 3 SO 3 + H 2 O H 2 SO 4 The overall reaction in presence of sunlight and relative humidity 30 -90% may be represented as: SO 2 + H 2 O H 2 SO 4 + O 3
Characteristics & Biochemical effects SO 2 is colourless , absorbs quickly and irritates the upper respiratory tract. Water-soluble gas with irritating odour. H 2 SO 4 formed destroys various functional molecules. Leads to breathlessness, impaired pulmonary function via airway resistance, impaired lung clearance and increased susceptibility for infection.
Control of SO 2 emissions from the anthropogenic activities (1) Removing SO 2 from flue gases before letting them out into the atmosphere (Lime stone) suggested to absorb SO 2 from the flue gases). 2 Ca. CO 3 + 2 SO 2 + O 2 Ca. SO 4 +CO 2 (2) Removing sulphur from the fuels , sulphur in coal can be removed by grinding and washing in coal washeries

Quiz • Enumerate only , all method for Control of SO 2 emissions from the anthropogenic activities • SO 2 is oxidised to SO 3 in atmospheric air by photolytic and catalytic processes, explain these by writing chemical reaction?
(3) Synthesis micro-organisms using bio-technology, which are capable of converting bound sulphur into soluble form (4) Generation of power by alternative energy sources
Acid rain Rain tends to be naturally acidic with a PH of 5. 6 - 5. 7 due to the reaction of atmospheric CO 2 with water to produce carbonic acid. This small amount of acidity is sufficient to dissolve minerals in the earth's crust and make them available to plant and animal life.

Over the last few decade : the rain pass through an atmosphere polluted with SOx & NOx. The falling rain react with these oxide pollutants to produce often a mixture of sulphuric acid, nitric acid and this is known as acid rain. The contributions from the three acids in the "acid rain" are mostly in the order: H 2 SO 4 > HNO 3 > HCI
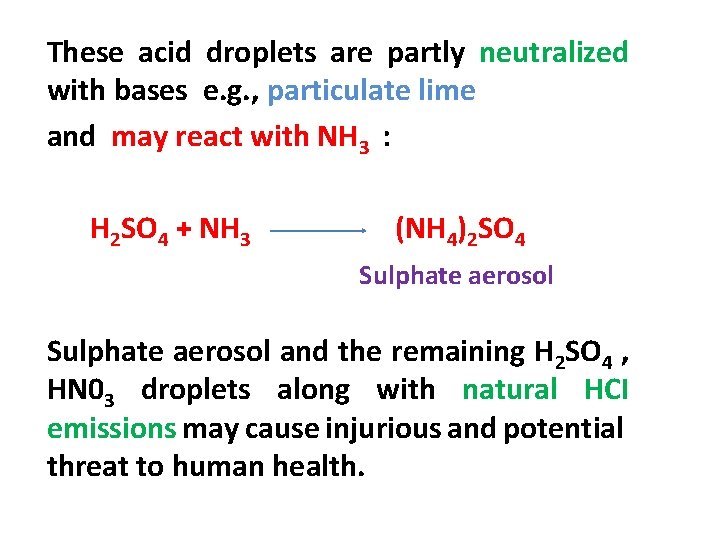
These acid droplets are partly neutralized with bases e. g. , particulate lime and may react with NH 3 : H 2 SO 4 + NH 3 (NH 4)2 SO 4 Sulphate aerosol and the remaining H 2 SO 4 , HN 03 droplets along with natural HCI emissions may cause injurious and potential threat to human health.
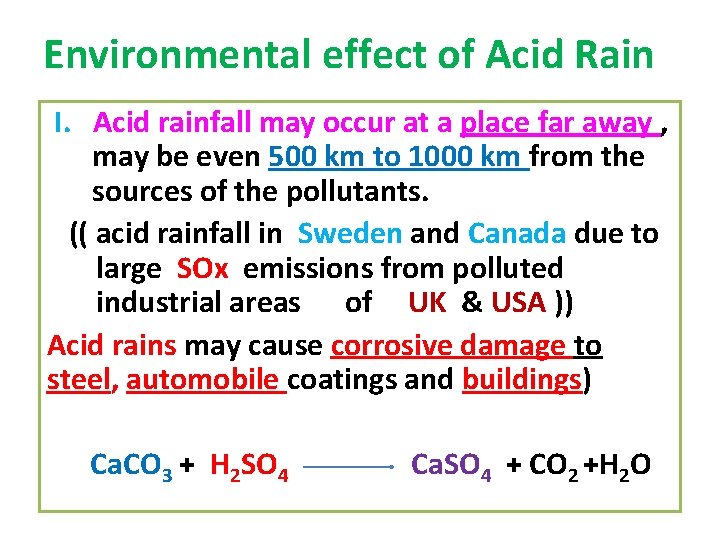
Environmental effect of Acid Rain I. Acid rainfall may occur at a place far away , may be even 500 km to 1000 km from the sources of the pollutants. (( acid rainfall in Sweden and Canada due to large SOx emissions from polluted industrial areas of UK & USA )) Acid rains may cause corrosive damage to steel, automobile coatings and buildings) Ca. CO 3 + H 2 SO 4 Ca. SO 4 + CO 2 +H 2 O

II. Acidification of soils , microbial and fixation of nitrogen due to alteration in soil chemistry leading to reduced forest productivity. III. Foliar damage to crops, leaching of nutrients from leaves, damage to young growing plant tissues and the process of photosynthesis

IV. Potential effects on aquatic systems, such as decreased in alkalinity , decline in productivity of fish etc. V. Possible effects on humans. Lungs, skin, hair may be affected. VI. Acidification of the lakes-Water may increases in heavy metal concentrations , exceed public health limits, may cause injurious and potential threat to human health.
- Slides: 35