Carbon Dioxide Adsorption Comparative Analysis JerEco Basic information

Carbon Dioxide Adsorption: Comparative Analysis Jer-Eco
Basic information of the burning of fossil fuel to produce electricity CO 2 emissions from energy production has been one of the main contributors towards global warming: a change in the atmosphere that can interrupt the delicate equilibrium needed in order for earth to function properly. With electricity contributing approx 34% of the carbon emissions in the US, it makes the electric industry a prime target in regards of controlling CO 2 emissions. With renewable energy still well into the future, CO 2 absorption proves to be the most optimal method for CO 2 control. Three different absorption methods are currently proposed: Alumina, Zeolites, and Carbon-Carbon Fiber Composite Molecular Sieve (CFCMS), all of which function differently in regards to CO 2 absorption. In this review, we will analyze the function of each of the methods, using comparative analysis to assess the optimal environments for each of the listed methods.

Absorption Process

Alumina • • • Also known as aluminum oxide Will tend to adsorb more CO 2 if exposed to lower concentrations of CO 2 Will tend to perform better if pretreated with helium @ high temperatures (preferably at 140ºC) o eliminates moisture, gases, and volatiles Feed flow rate of entire experiment was maintained at 52 m. L/min (8 m. L/min of CO 2 and 44 m. L/min of nitrogen) o Nitrogen will increase adsorption because it increases the basicity o Carbon dioxide is an acidic gas --> opposites attract Average pore size- 49. 50 A (nanometers) o responsible for the high surface area of mesoporous alumina
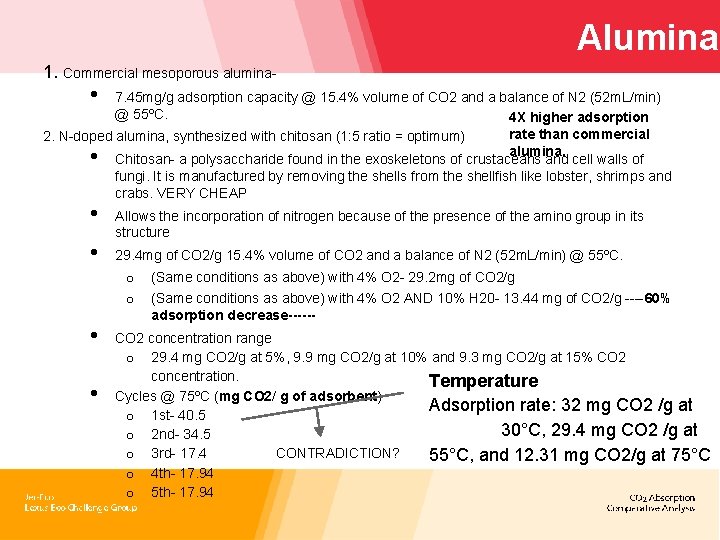
Alumina 1. Commercial mesoporous alumina- • 7. 45 mg/g adsorption capacity @ 15. 4% volume of CO 2 and a balance of N 2 (52 m. L/min) @ 55ºC. 4 X higher adsorption rate than commercial 2. N-doped alumina, synthesized with chitosan (1: 5 ratio = optimum) alumina. Chitosan- a polysaccharide found in the exoskeletons of crustaceans and cell walls of fungi. It is manufactured by removing the shells from the shellfish like lobster, shrimps and crabs. VERY CHEAP • • • Allows the incorporation of nitrogen because of the presence of the amino group in its structure 29. 4 mg of CO 2/g 15. 4% volume of CO 2 and a balance of N 2 (52 m. L/min) @ 55ºC. o o • • (Same conditions as above) with 4% O 2 - 29. 2 mg of CO 2/g (Same conditions as above) with 4% O 2 AND 10% H 20 - 13. 44 mg of CO 2/g ----60% adsorption decrease------ CO 2 concentration range o 29. 4 mg CO 2/g at 5%, 9. 9 mg CO 2/g at 10% and 9. 3 mg CO 2/g at 15% CO 2 concentration. Temperature Cycles @ 75ºC (mg CO 2/ g of adsorbent) Adsorption rate: 32 mg CO 2 /g at o 1 st- 40. 5 30°C, 29. 4 mg CO 2 /g at o 2 nd- 34. 5 CONTRADICTION? o 3 rd- 17. 4 55°C, and 12. 31 mg CO 2/g at 75°C o 4 th- 17. 94 o 5 th- 17. 94
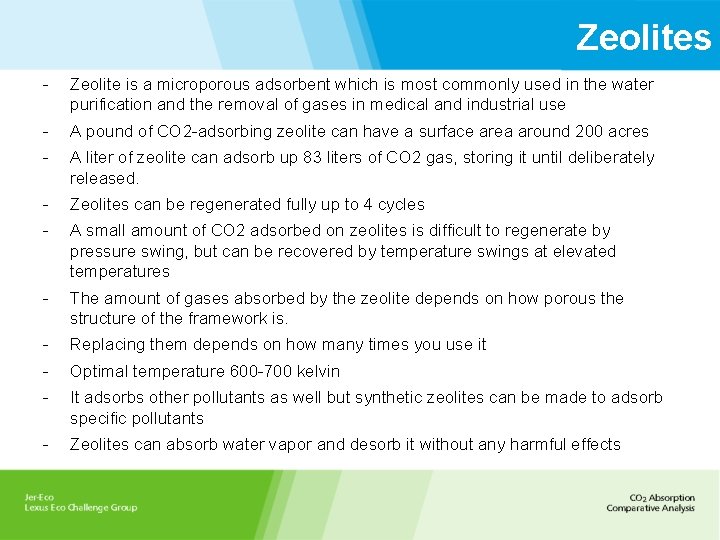
Zeolites - Zeolite is a microporous adsorbent which is most commonly used in the water purification and the removal of gases in medical and industrial use - A pound of CO 2 -adsorbing zeolite can have a surface area around 200 acres - A liter of zeolite can adsorb up 83 liters of CO 2 gas, storing it until deliberately released. - Zeolites can be regenerated fully up to 4 cycles - A small amount of CO 2 adsorbed on zeolites is difficult to regenerate by pressure swing, but can be recovered by temperature swings at elevated temperatures - The amount of gases absorbed by the zeolite depends on how porous the structure of the framework is. - Replacing them depends on how many times you use it - Optimal temperature 600 -700 kelvin - It adsorbs other pollutants as well but synthetic zeolites can be made to adsorb specific pollutants - Zeolites can absorb water vapor and desorb it without any harmful effects

Zeolites - If we can apply them at the top of smokestacks of huge industrial plants, much of the pollutants being released into the air will be adsorbed by the zeolites. Then, after the zeolites become full, we can make the zeolites desorb the materials. After about 4 times of desorbing, it won't be able to perfectly desorb all materials. But that amount is miniscule. I think we might also be able to grind up zeolites and put them in major roads or put them in the sound barriers next to the highways so they can soak up all the pollutants car exhaust has - The cost if relatively low for natural zeolites($6. 50 to $40. 00 per pound) but they are not very pure and synthetic(up to $100 per pound) zeolites are more expensive and pure because companies charge a lot for them - Synthetic zeolites have an advantage because they can be produced in uniform shapes and structures - Synthetic zeolite can also be produced in new structures not found in nature - Synthetic zeolites are easy to produce because the main two products in zeolites are alumina and silica, which are abundant minerals in the world
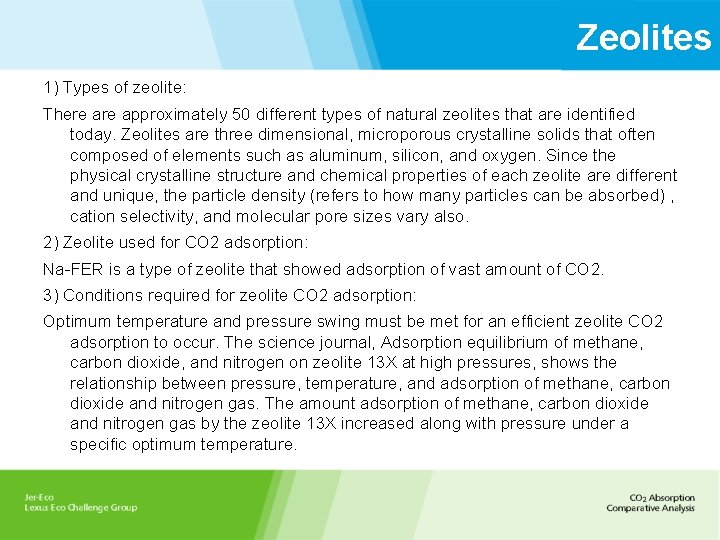
Zeolites 1) Types of zeolite: There approximately 50 different types of natural zeolites that are identified today. Zeolites are three dimensional, microporous crystalline solids that often composed of elements such as aluminum, silicon, and oxygen. Since the physical crystalline structure and chemical properties of each zeolite are different and unique, the particle density (refers to how many particles can be absorbed) , cation selectivity, and molecular pore sizes vary also. 2) Zeolite used for CO 2 adsorption: Na-FER is a type of zeolite that showed adsorption of vast amount of CO 2. 3) Conditions required for zeolite CO 2 adsorption: Optimum temperature and pressure swing must be met for an efficient zeolite CO 2 adsorption to occur. The science journal, Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13 X at high pressures, shows the relationship between pressure, temperature, and adsorption of methane, carbon dioxide and nitrogen gas. The amount adsorption of methane, carbon dioxide and nitrogen gas by the zeolite 13 X increased along with pressure under a specific optimum temperature.
Activated Carbon- Carbon Fiber Composite Molecular Sieve (CFCMS) Physical characteristics of CFCMS is a carbon adsorbent material made of petroleum-derived isotropic pitch carbon fibers and phenolic-resin-derived carbon binder. It is characterized by its rigid, strong monolithic structure which is resistant to attrition and dusting. CFCMS also has a kinetic advantage due to small fiber diameter (8 -16 µm) and because of its continuous carbon skeletal structure, electrically conductive. The monolith is macroporous, with large pores between fibers. However, it is also highly microporous, due to the connected network of micropores within the carbon fibers. This open structure allows the free flow of fluids through the material such that gases can reach the micropores where they will be selectively adsorbed. CFCMS has been highly successful in the adsorption of polar molecules and some nonpolar, flat molecules such as CO 2. CFCMS can be mesoporous and microporous, and can have a density of anywhere between. 2 -. 4 g/cm 3,
CFCMS novel desorption process. The electrical conductivity of the material due to the continuous nature of its carbon skeletal structure, lead to the development of a novel desorption process, "electrical swing adsorption. " CO 2 absorption rate and conditions for CFCMS can absorb more than 50 cm 2/g, or 100 mg/g of CO 2 at 303 K and atmospheric pressure. This figure decreases as the temperature increases, and at 373 K, this figure is 20 cm 2/g, or 40 mg/g. However, as pressure increases, the absorption rate increases. At 298 K and at 57. 24 atm, the CFCMS can absorb more than 490 mg/g. CFCMS can also rapidly desorb CO 2 if a low current is applied.

Sources http: //www. netl. doe. gov/publications/proceedings/01/carbon_seq/3 b 3. pdf http: //onlinelibrary. wiley. com/doi/10. 1002/cssc. 200900036/full Cavenati, S. , Grande, C. A. , & Rodrigues, A. E. (2004). Adsorption equilibrium of methane, carbon dioxide, and nitrogen. American Chemical Society, 49(2004), 1095 -1101. doi: 10. 1021/je 0498917 Yang, S. T. , Kim, J. , & Ahn, W. S. (2010). CO 2 adsorption over ion-exchanged zeolite beta with alkali. Microporous and Mesoporous Materials, 135(2010), 90 -94. doi: 10. 1016/j. micromeso. 2010. 06. 015 http: //www. epa. gov/climatechange/ghgemissions/sources/electricity. html http: //www. netl. doe. gov/publications/proceedings/01/carbon_seq/3 b 1. pdf http: //www. netl. doe. gov/publications/proceedings/03/materials/burchell. pdf http: //www. osti. gov/energycitations/servlets/purl/385478 l 9 h. Qa. K/webviewable/Anovelcarbonfiberbasedmaterialandseparationtechnology. pdf http: //www. scribd. com/doc/47100584/36/Carbon-Fiber-Composite-Molecular-Sieves
- Slides: 11