Carbon Compounds Review Four major elements for life

Carbon Compounds Review Four major elements for life: C – Carbon H – Hydrogen O – Oxygen N - Nitrogen Gases
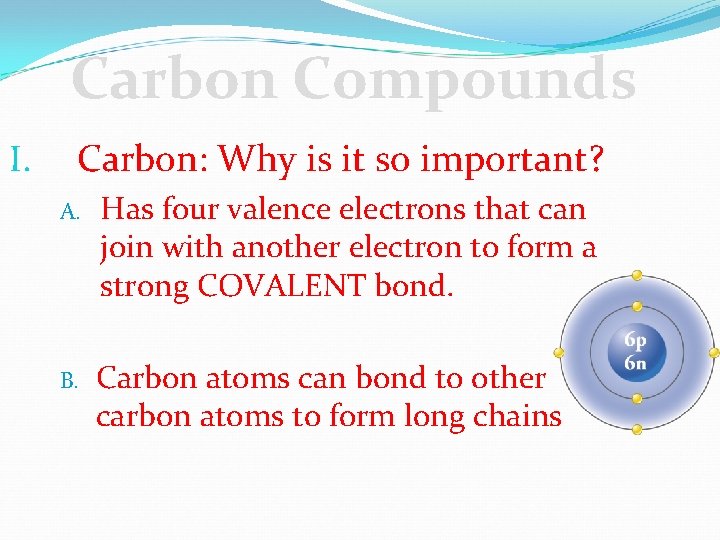
Carbon Compounds I. Carbon: Why is it so important? A. Has four valence electrons that can join with another electron to form a strong COVALENT bond. B. Carbon atoms can bond to other carbon atoms to form long chains

Carbon Compounds C. Carbon bonds: Up to four bonds for each C Single Double Triple
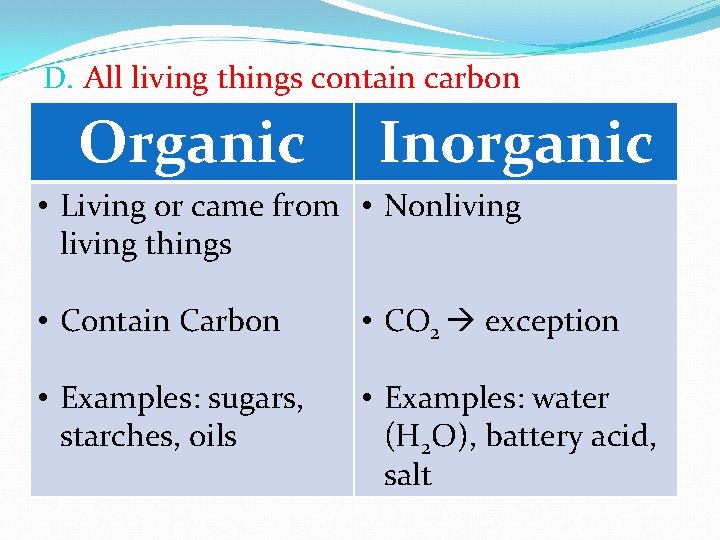
D. All living things contain carbon Organic Inorganic • Living or came from • Nonliving things • Contain Carbon • CO 2 exception • Examples: sugars, starches, oils • Examples: water (H 2 O), battery acid, salt

Carbon Compounds Carbon-based molecules are called organic compounds. Hydrocarbons – compounds composed of ONLY carbon and hydrogen Let’s build some hydrocarbons!
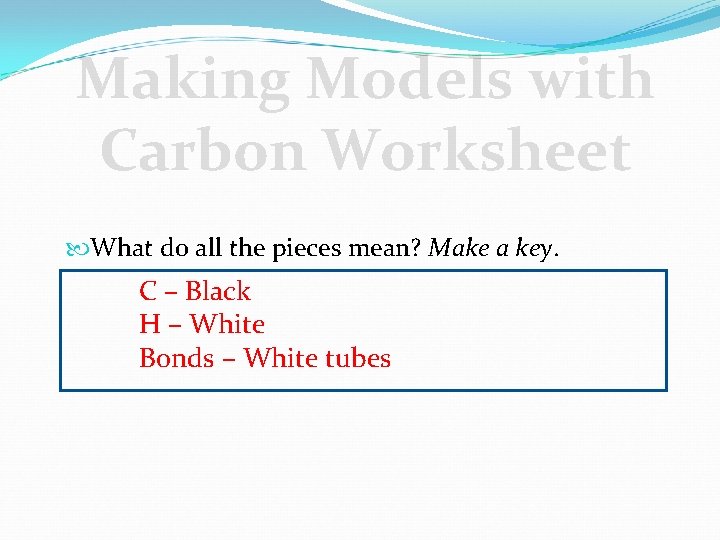
Making Models with Carbon Worksheet What do all the pieces mean? Make a key. C – Black H – White Bonds – White tubes
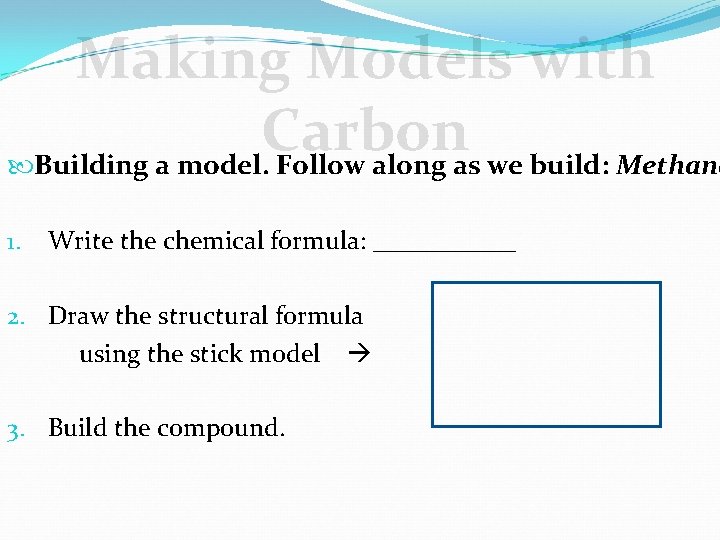
Making Models with Carbon Building a model. Follow along as we build: Methane 1. Write the chemical formula: ______ 2. Draw the structural formula using the stick model 3. Build the compound.
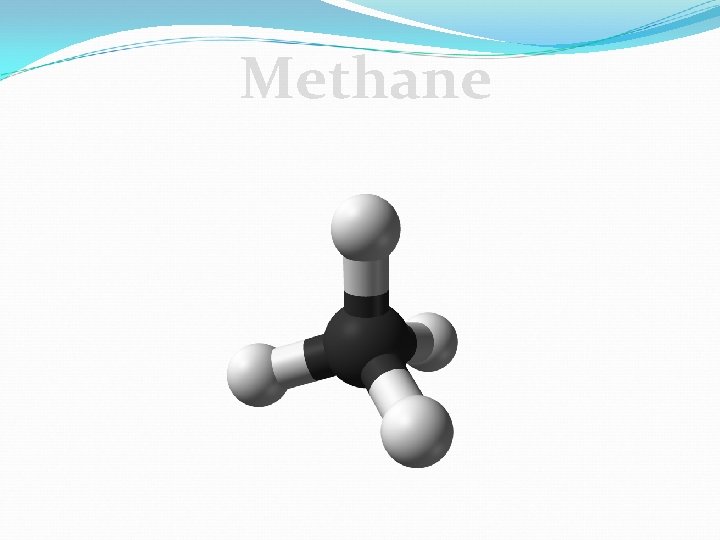
Methane
On your own try to draw and build the following molecules: Ethane: Propane: Butane: Octane:
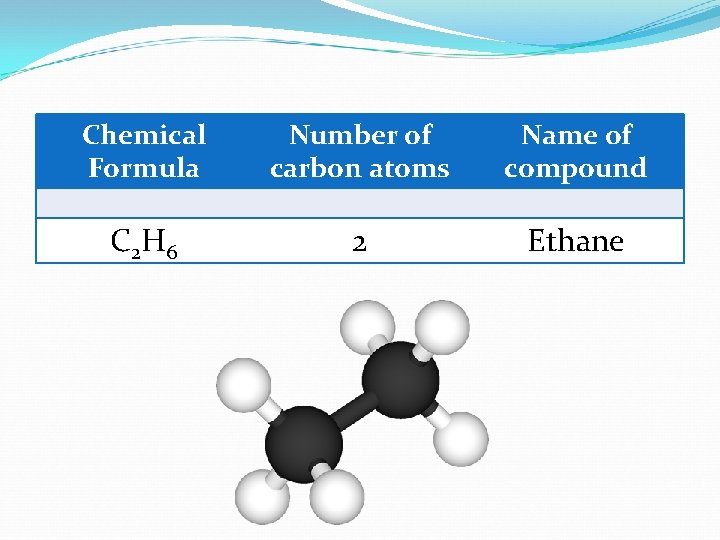
Chemical Formula Number of carbon atoms Name of compound C 2 H 6 2 Ethane
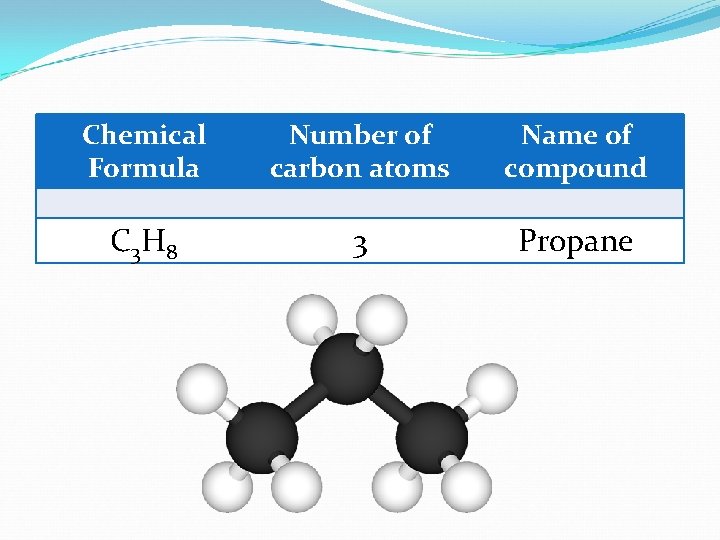
Chemical Formula Number of carbon atoms Name of compound C 3 H 8 3 Propane
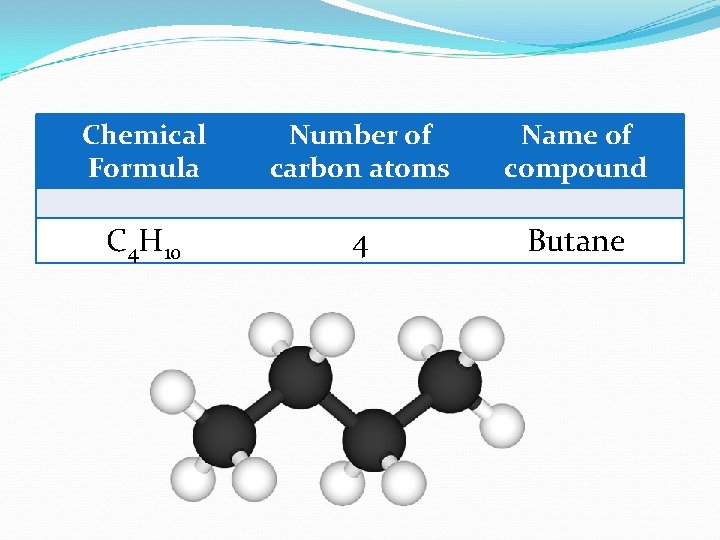
Chemical Formula Number of carbon atoms Name of compound C 4 H 10 4 Butane
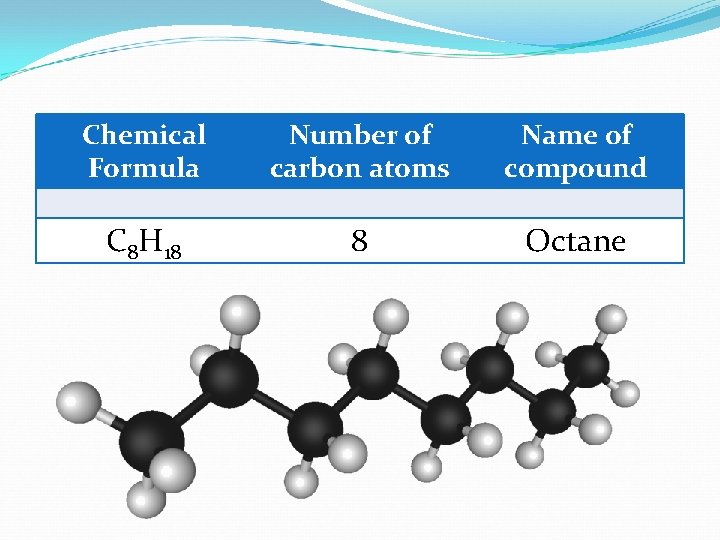
Chemical Formula Number of carbon atoms Name of compound C 8 H 18 8 Octane

Try these questions on your own: How many bonds can a hydrogen atom from? How many bonds can a carbon atom form? Notice that all of the hydrocarbons we have built end with –ane. (i. e. propane) The suffix –ane means that a hydrocarbon only contains single bonds between the carbon atoms. We call these hydrocarbon alkanes.
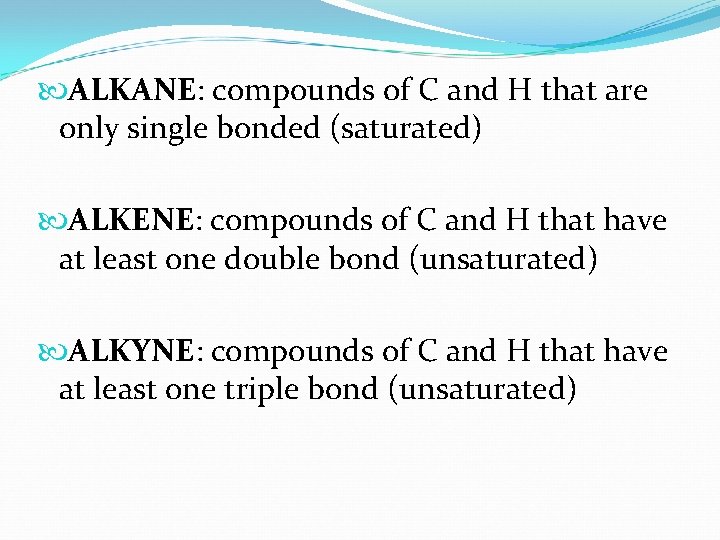
ALKANE: compounds of C and H that are only single bonded (saturated) ALKENE: compounds of C and H that have at least one double bond (unsaturated) ALKYNE: compounds of C and H that have at least one triple bond (unsaturated)
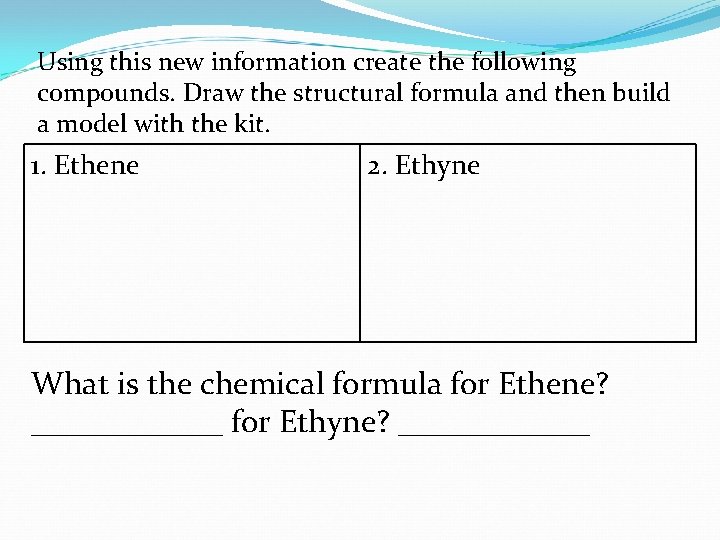
Using this new information create the following compounds. Draw the structural formula and then build a model with the kit. 1. Ethene 2. Ethyne What is the chemical formula for Ethene? ______ for Ethyne? ______
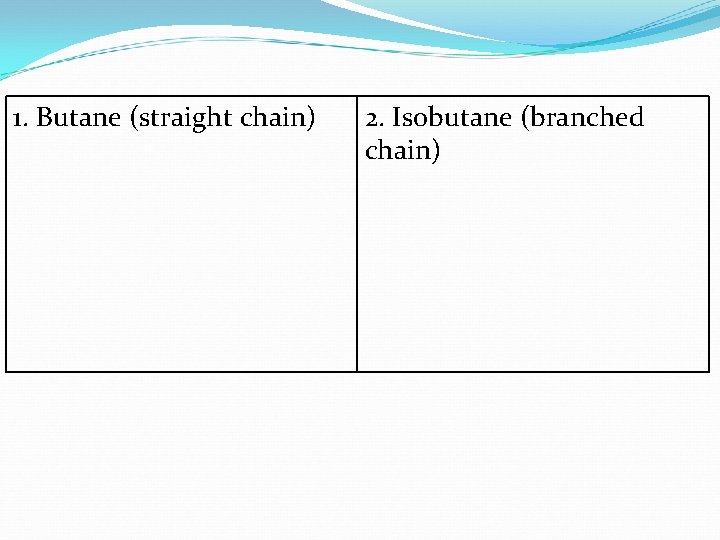
1. Butane (straight chain) 2. Isobutane (branched chain)
Macromolecules II. Macromolecules – Four main groups of organic compounds formed by polymerization large compounds are made by joining smaller ones (monomers) together.
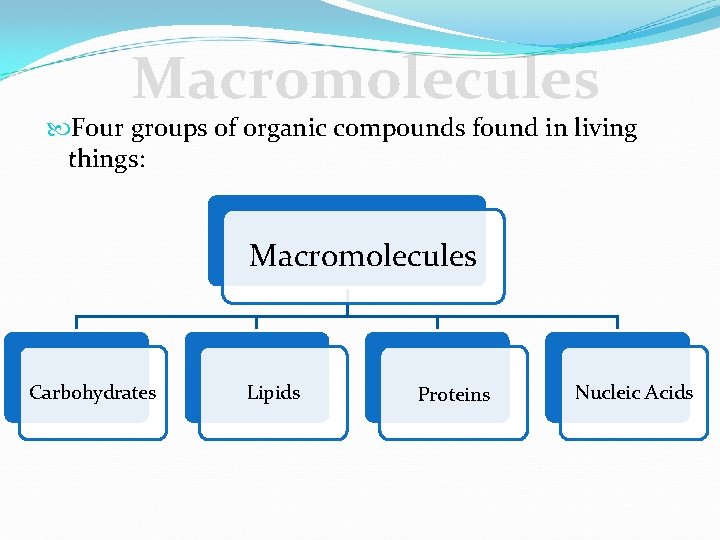
Macromolecules Four groups of organic compounds found in living things: Macromolecules Carbohydrates Lipids Proteins Nucleic Acids

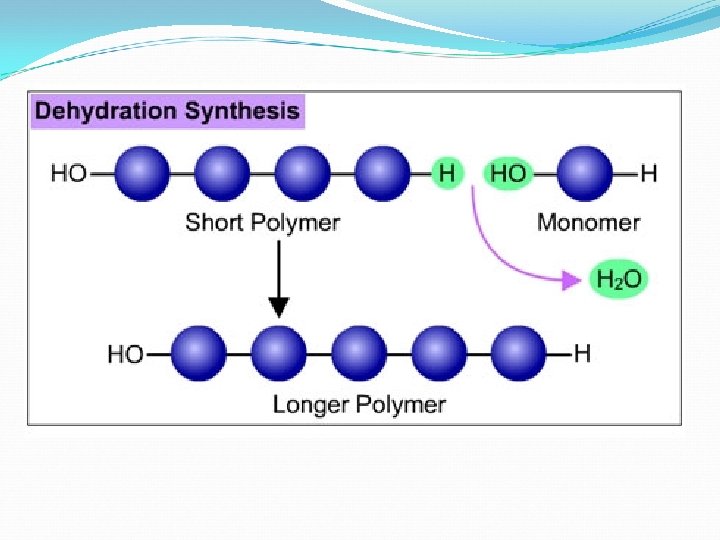
How to Make a Macromolecule ØCondensation reaction (dehydration synthesis) How monomers are linked together. For each monomer added to a chain, one water molecule (H 2 O) is removed.

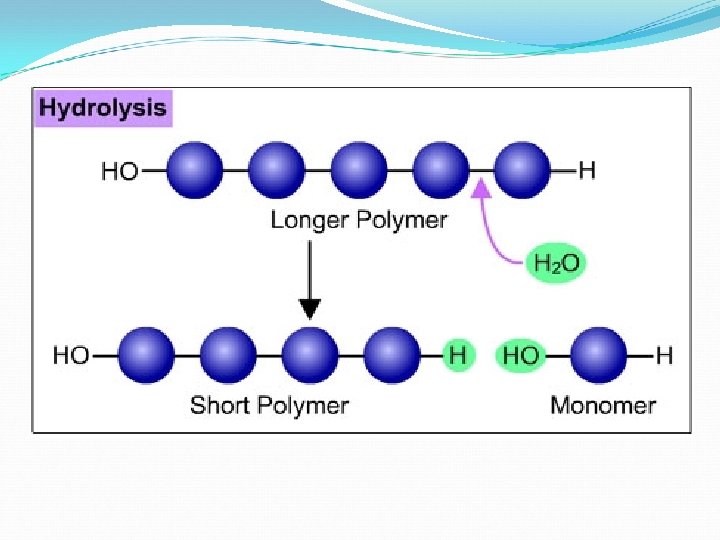
How to Break a Macromolecule ØHydrolysis Breaking polymers into monomers using/adding water. We have to break down our food to get energy!
Carbohydrates A. Carbohydrates v Monomer- Monosaccharides (Single sugar molecules) v Characteristics: Living things use carbs as their main source of energy. Plants and some animals use carbs for structure.
Carbohydrates Examples: Glucose – main product of photosynthesis Galactose – component of milk Fructose – component of fruit
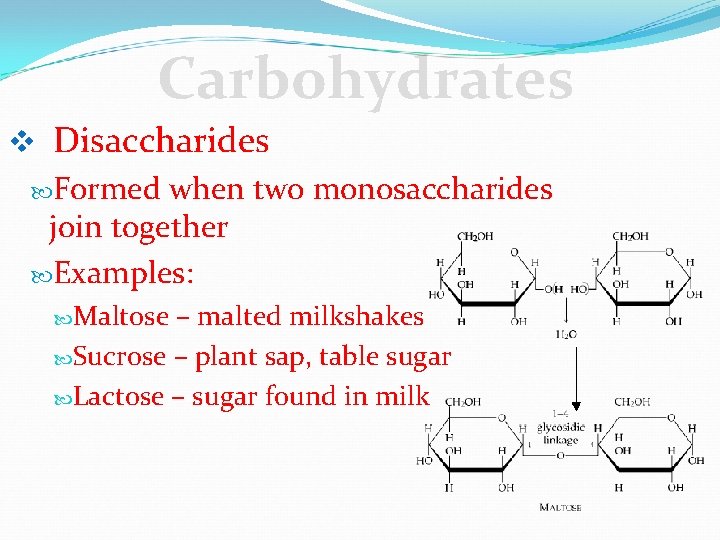
Carbohydrates v Disaccharides Formed when two monosaccharides join together Examples: Maltose – malted milkshakes Sucrose – plant sap, table sugar Lactose – sugar found in milk
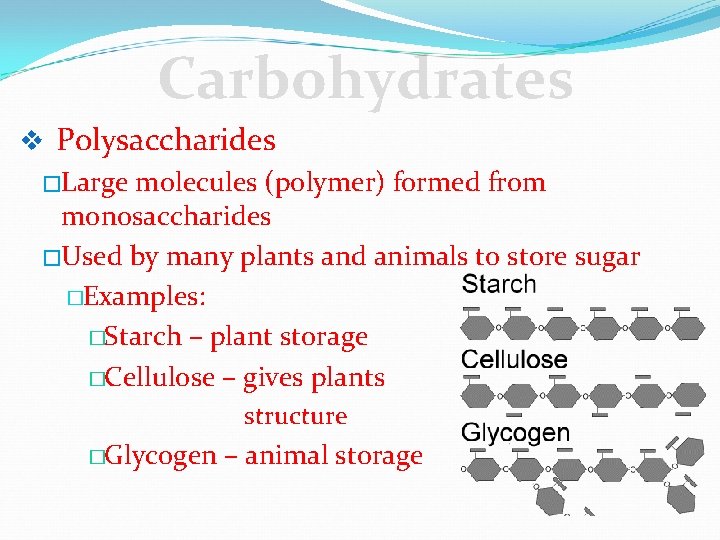
Carbohydrates v Polysaccharides �Large molecules (polymer) formed from monosaccharides �Used by many plants and animals to store sugar �Examples: �Starch – plant storage �Cellulose – gives plants structure �Glycogen – animal storage

Let’s Build Some Carbohydrates!
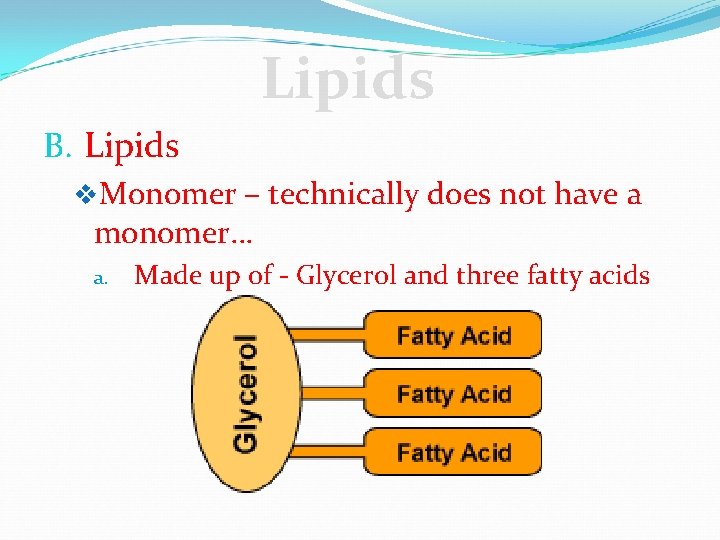
Lipids B. Lipids v. Monomer – technically does not have a monomer… a. Made up of - Glycerol and three fatty acids

Lipids v. Characteristics Widely varied in structure and function Substances that are insoluble in a polar solvent and soluble in a nonpolar solvent. q Lipids cannot dissolve in water.
Lipids v Importance: Long-term energy Insulation and protection Chemical messengers; surround nerve cells; myelin Cell membranes
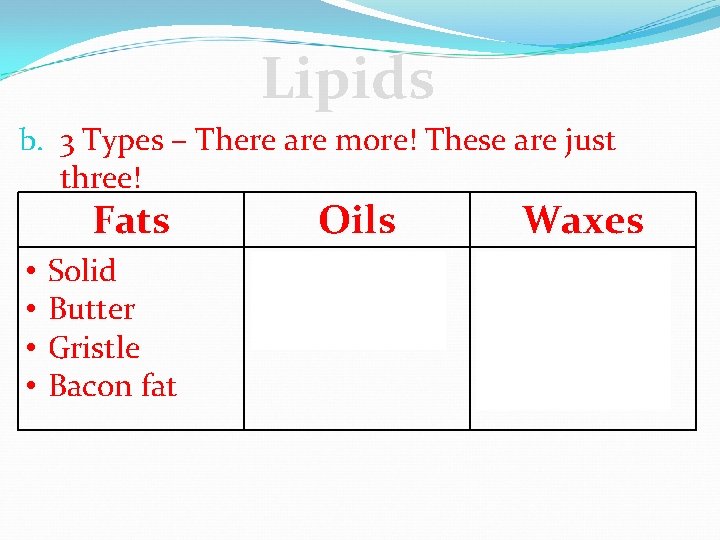
Lipids b. 3 Types – There are more! These are just three! • • Fats Oils Waxes Solid Butter Gristle Bacon fat • Liquid • Olive Oil • Hard Solid • Candles • Surfboard wax
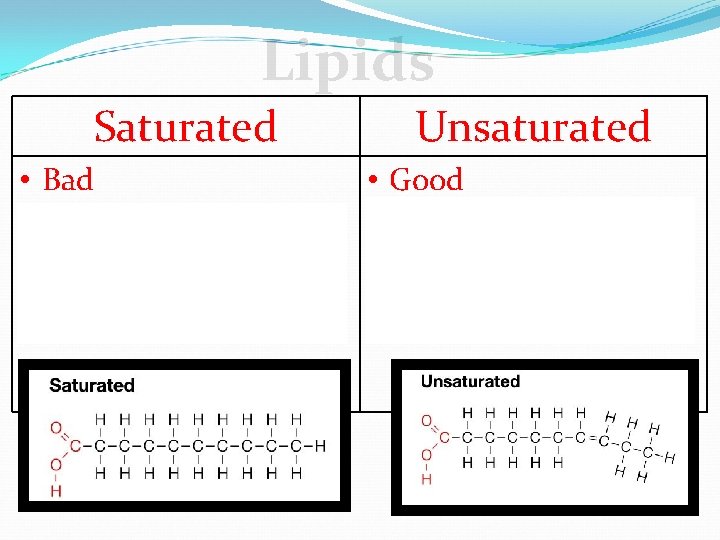
Lipids Saturated • • Bad Solid animal fats Causes heart disease Single bonds between carbons Unsaturated • • Good Fish Oil Prevents heart disease Double bonds between carbons

Let’s Build Some Lipids!
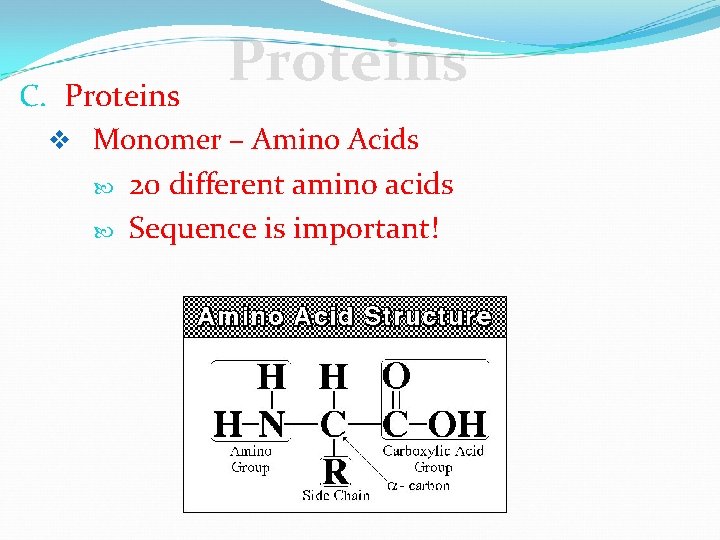
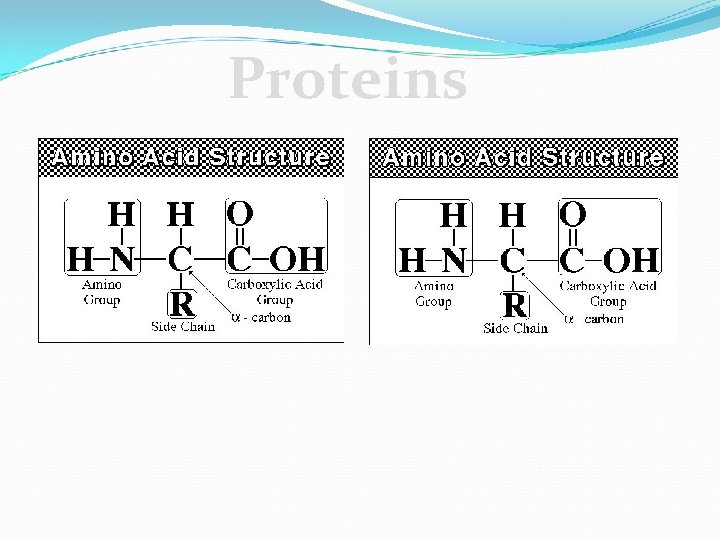
Proteins C. Proteins v Monomer – Amino Acids 20 different amino acids Sequence is important!
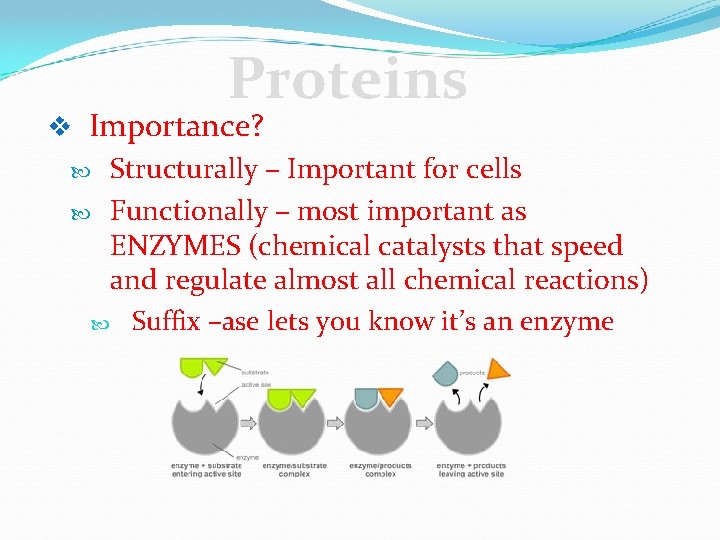
Proteins v Importance? Structurally – Important for cells Functionally – most important as ENZYMES (chemical catalysts that speed and regulate almost all chemical reactions) Suffix –ase lets you know it’s an enzyme
Proteins v Examples: 1. Structural Protein – found in hair and tendons/ligaments 2. Defensive Protein – Antibodies of the immune system 3. Signal Protein – hormones/messengers that communicate between cells

Proteins 4. Transport Proteins – ex/ transporting oxygen/sugars 5. Storage Proteins – ex/ store amino acids for embryos
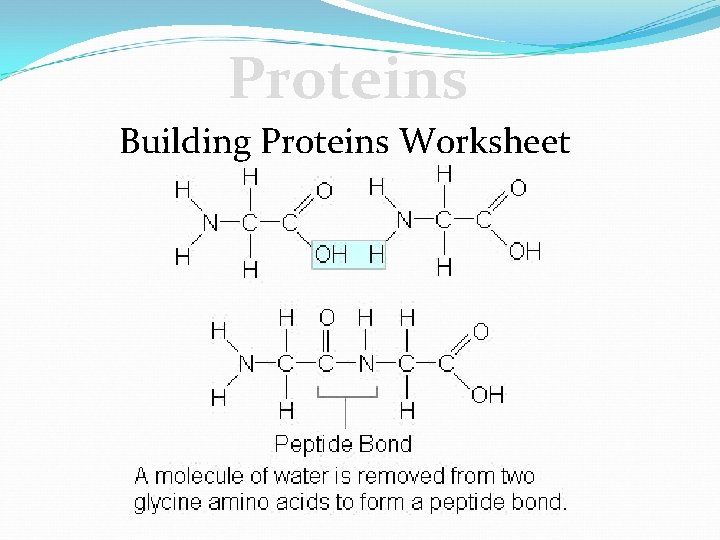
Proteins Building Proteins Worksheet
Proteins
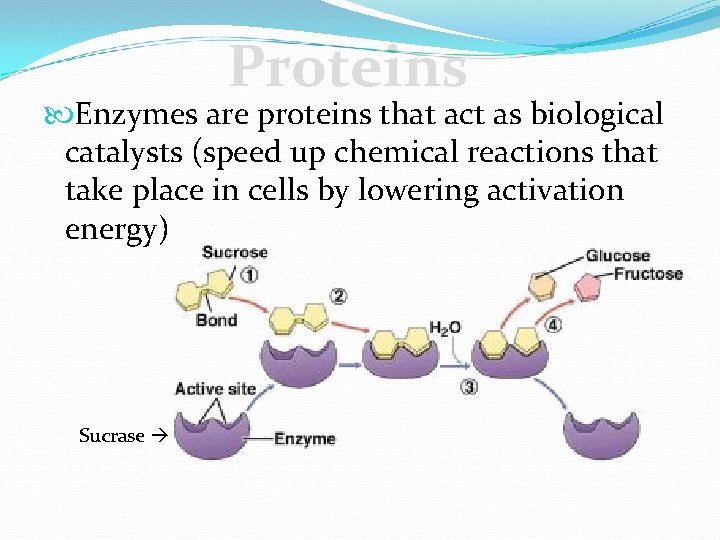
Proteins Enzymes are proteins that act as biological catalysts (speed up chemical reactions that take place in cells by lowering activation energy) Sucrase

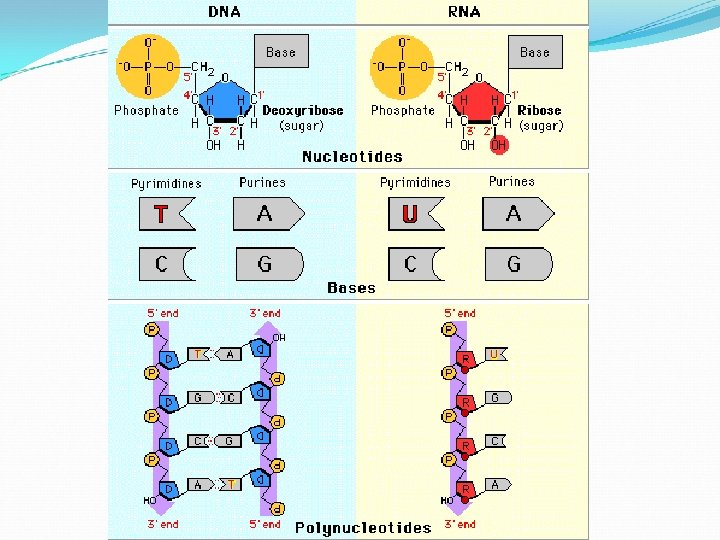
Nucleic Acids D. Nucleic Acids v Monomers (building blocks) • • Nucleotides Each nucleotide has three parts
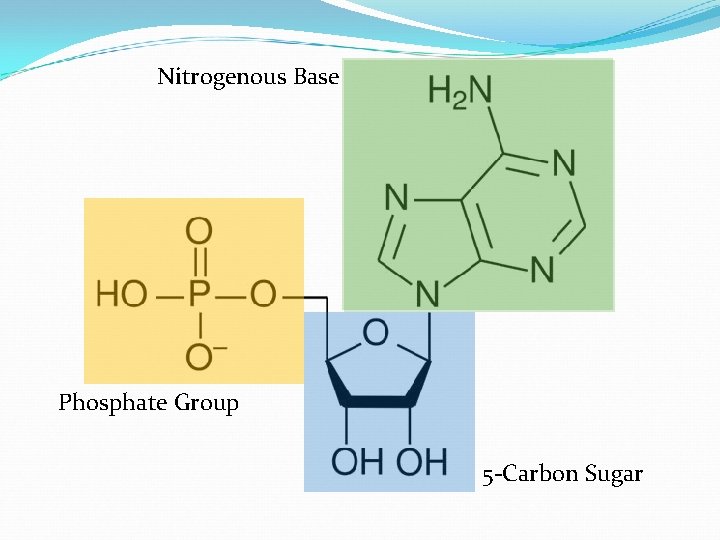
Nitrogenous Base Phosphate Group 5 -Carbon Sugar
Nucleic Acids v Importance? • Function - Store and transmit hereditary/genetic information v Two kinds Deoxyribonucleic acid (DNA) 2. Ribonucleic acid (RNA) 1.
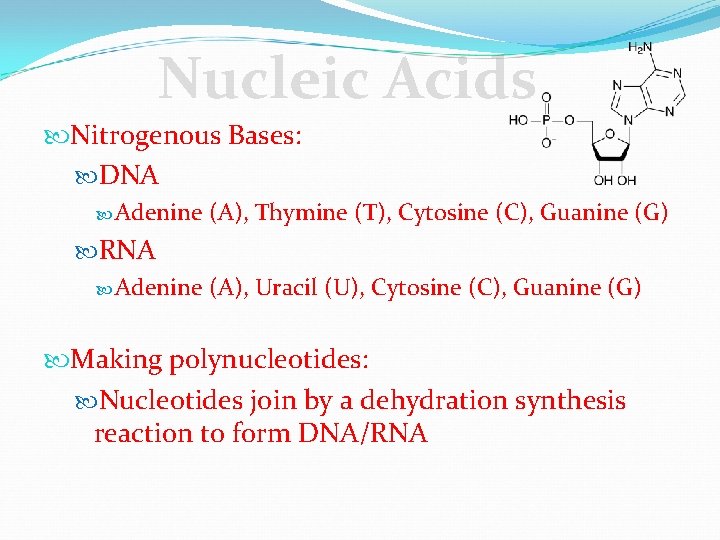
Nucleic Acids Nitrogenous Bases: DNA Adenine (A), Thymine (T), Cytosine (C), Guanine (G) RNA Adenine (A), Uracil (U), Cytosine (C), Guanine (G) Making polynucleotides: Nucleotides join by a dehydration synthesis reaction to form DNA/RNA
Protein Review What are the elements found in proteins? Functionally why are proteins important? What are the monomers of proteins? To join two amino acids together, what process has to occur? To break apart amino acids, what process has to occur?


Enzyme Activity Lab
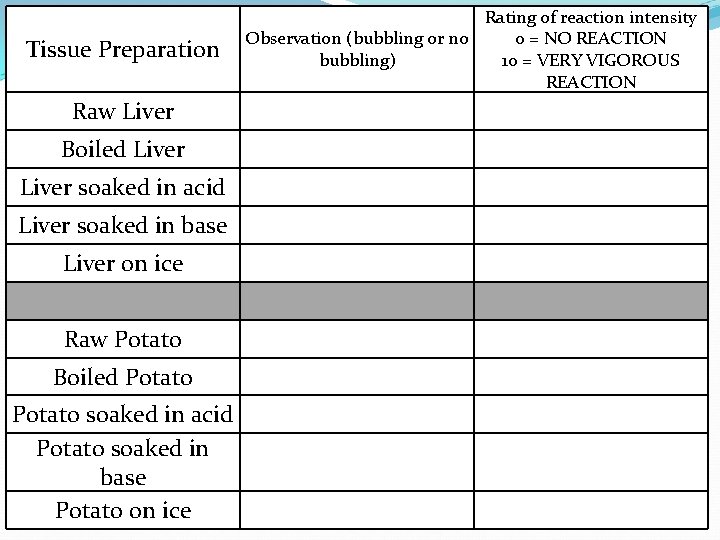
Tissue Preparation Rating of reaction intensity Observation (bubbling or no 0 = NO REACTION bubbling) 10 = VERY VIGOROUS REACTION Raw Liver Boiled Liver soaked in acid Liver soaked in base Liver on ice Raw Potato Boiled Potato Potato soaked in acid Potato soaked in base Potato on ice
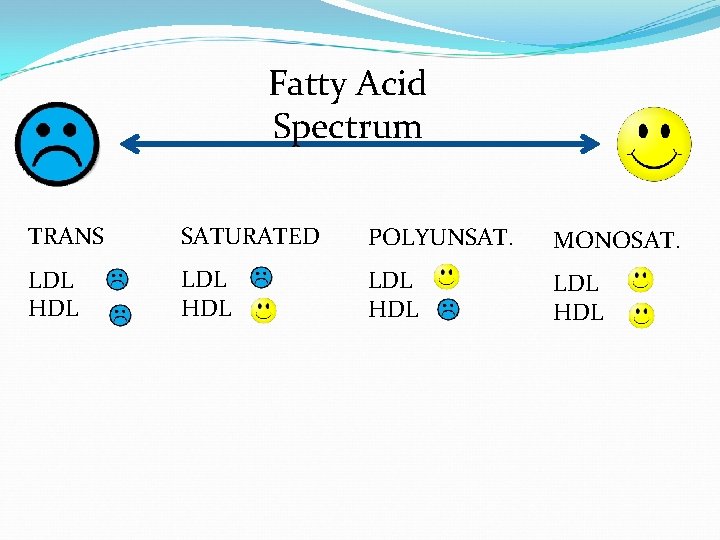
Fatty Acid Spectrum TRANS SATURATED POLYUNSAT. MONOSAT. LDL HDL
- Slides: 53