Carbon Compounds Organic Inorganic Made primarily of Carbon

Carbon Compounds

Organic Inorganic • Made primarily of Carbon • Makes up most living matter • Does not contain carbon atoms. (There are exceptions like CO 2) • Examples: sugars, starch, • Example: WATER
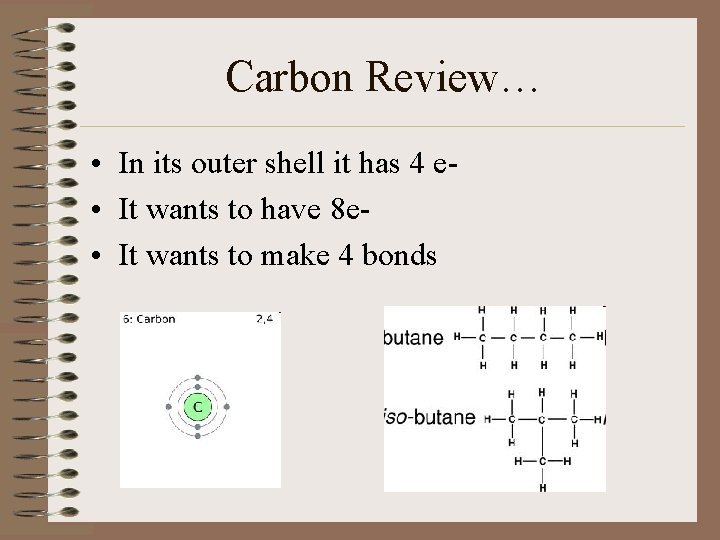
Carbon Review… • In its outer shell it has 4 e • It wants to have 8 e • It wants to make 4 bonds
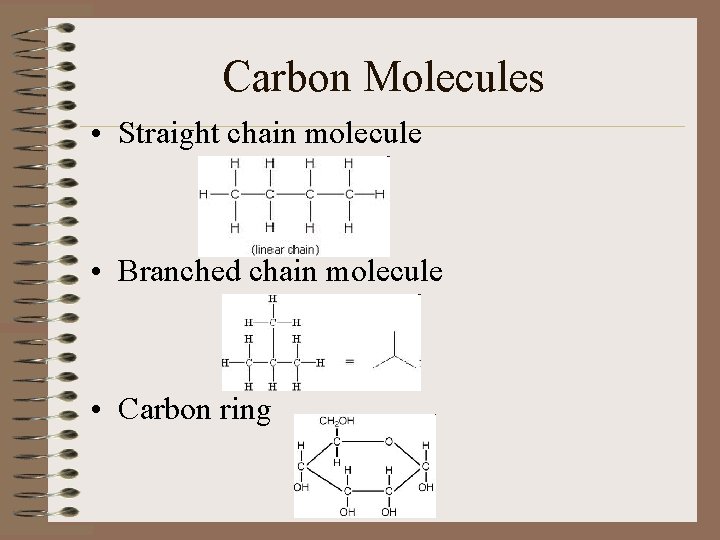
Carbon Molecules • Straight chain molecule • Branched chain molecule • Carbon ring
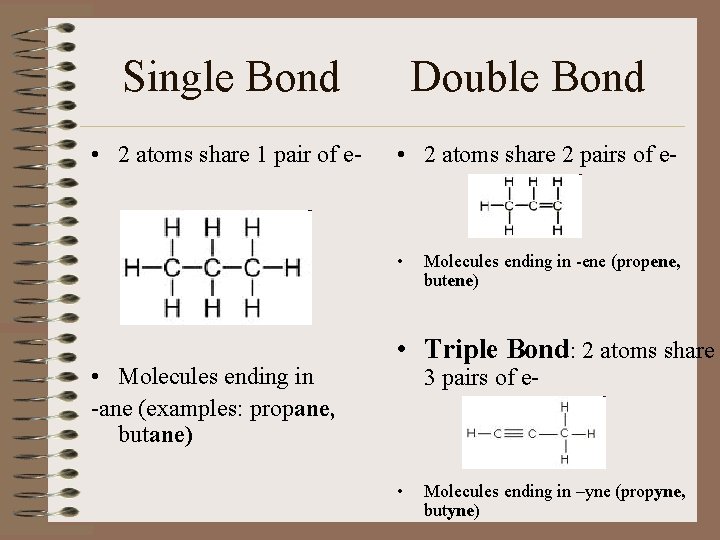
Single Bond • 2 atoms share 1 pair of e- Double Bond • 2 atoms share 2 pairs of e- • • Molecules ending in -ane (examples: propane, butane) Molecules ending in -ene (propene, butene) • Triple Bond: 2 atoms share 3 pairs of e- • Molecules ending in –yne (propyne, butyne)
Functional Groups • Clusters of atoms • They influence the characteristics of the molecule they compose • They influence the way the molecule will react with other molecules (bonding)
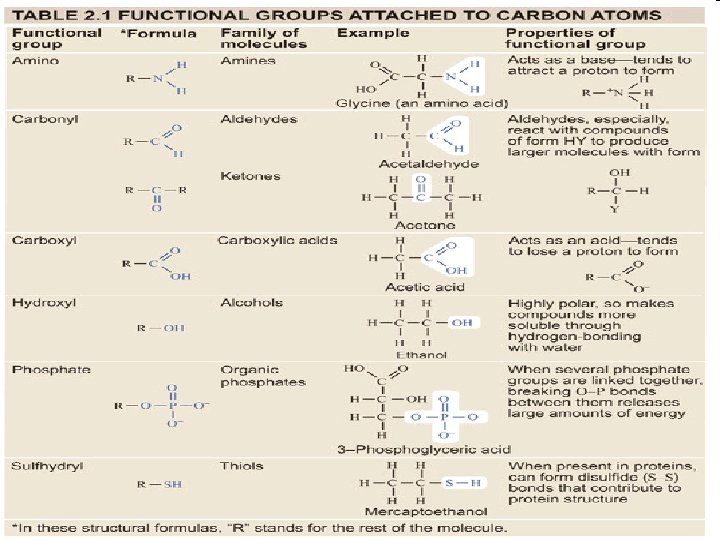
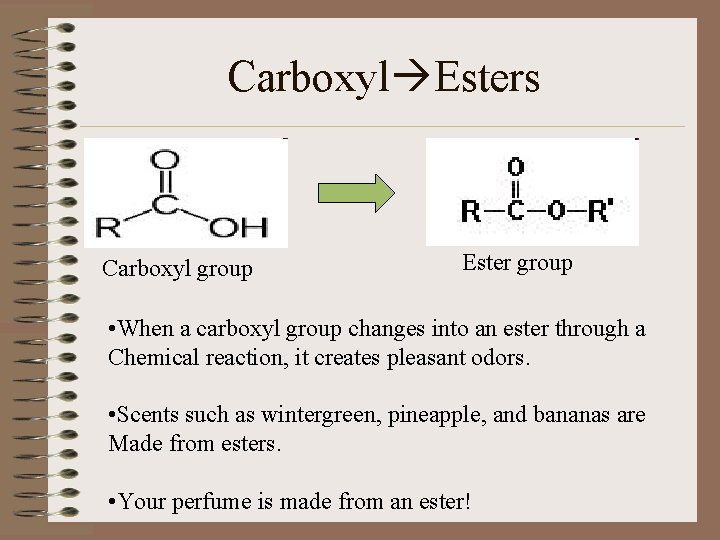
Carboxyl Esters Carboxyl group Ester group • When a carboxyl group changes into an ester through a Chemical reaction, it creates pleasant odors. • Scents such as wintergreen, pineapple, and bananas are Made from esters. • Your perfume is made from an ester!
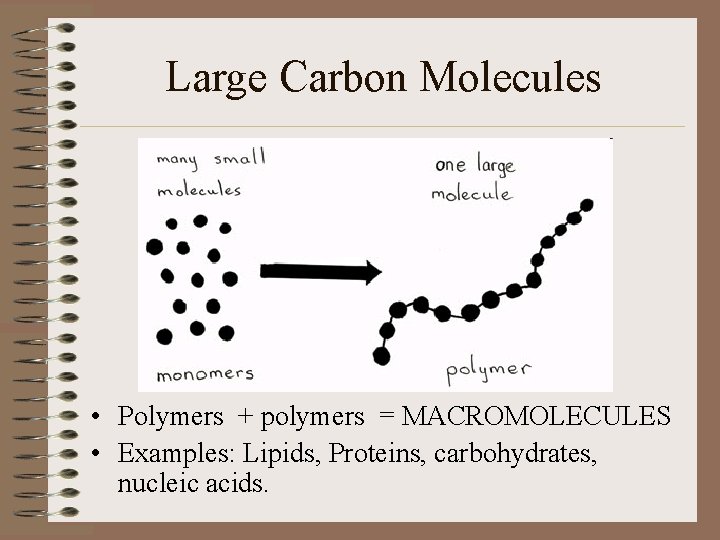
Large Carbon Molecules • Polymers + polymers = MACROMOLECULES • Examples: Lipids, Proteins, carbohydrates, nucleic acids.
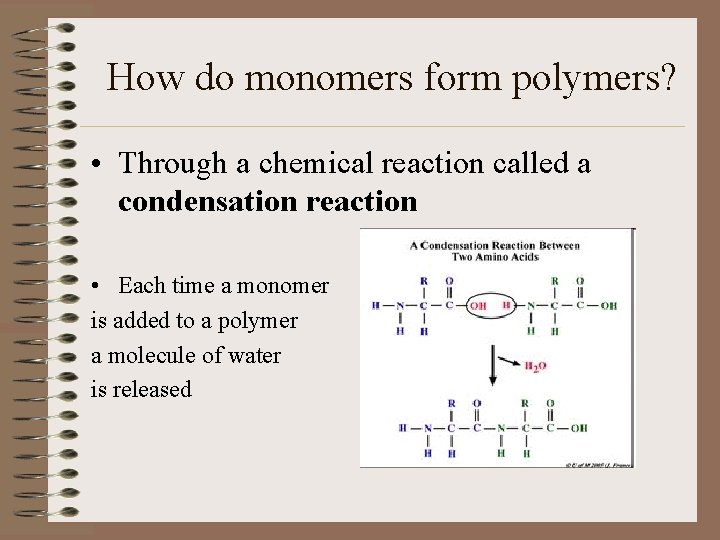
How do monomers form polymers? • Through a chemical reaction called a condensation reaction • Each time a monomer is added to a polymer a molecule of water is released
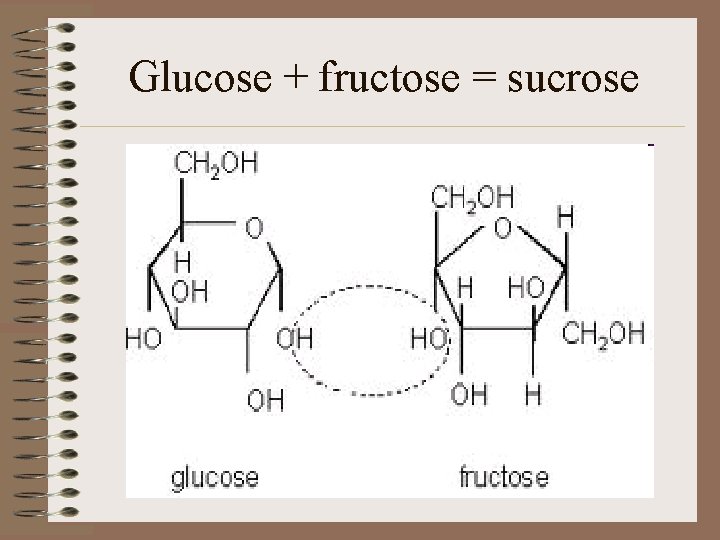
Glucose + fructose = sucrose
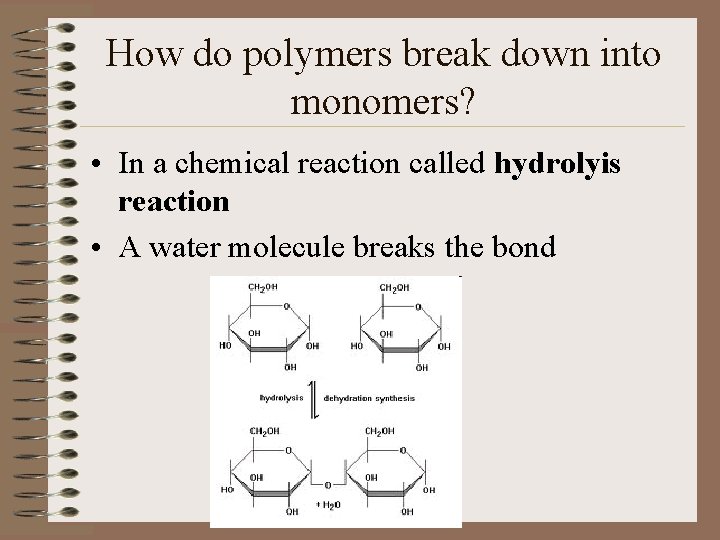
How do polymers break down into monomers? • In a chemical reaction called hydrolyis reaction • A water molecule breaks the bond
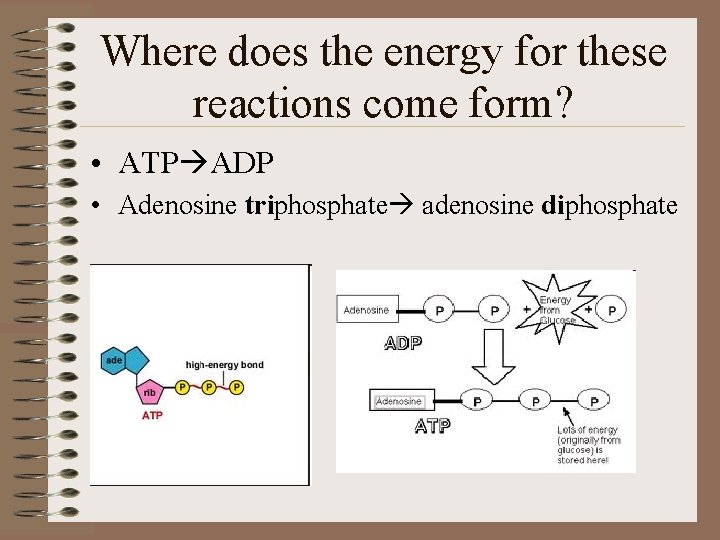
Where does the energy for these reactions come form? • ATP ADP • Adenosine triphosphate adenosine diphosphate
- Slides: 13