Carbon Compounds in Cells Chapter 3 Importance of

Carbon Compounds in Cells Chapter 3

Importance of Carbon permeates the world of life— from the energy-requiring activities and structural organization of cells, to physical and chemical conditions that span the globe and influence ecosystems everywhere.

Humans and Global Warming • Fossil fuels are rich in carbon • Use of fossil fuels releases CO 2 into atmosphere • Increased CO 2 may contribute to global warming

Organic Compounds Hydrogen and other elements covalently bonded to carbon Carbohydrates Lipids Proteins Nucleic Acids
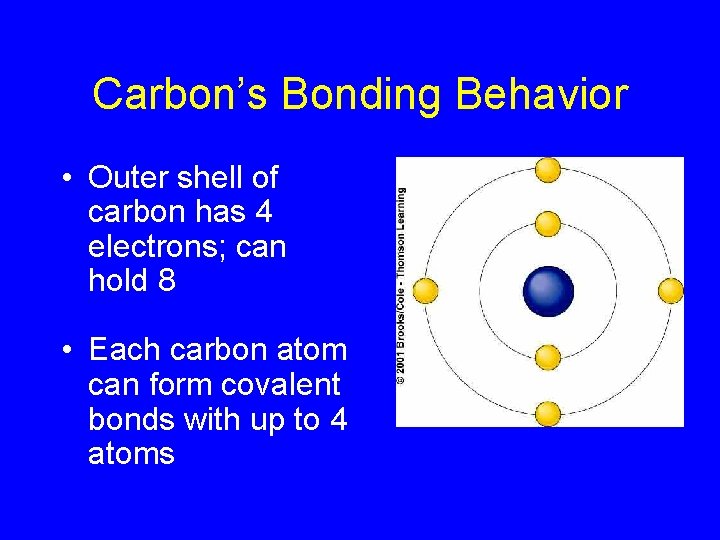
Carbon’s Bonding Behavior • Outer shell of carbon has 4 electrons; can hold 8 • Each carbon atom can form covalent bonds with up to 4 atoms
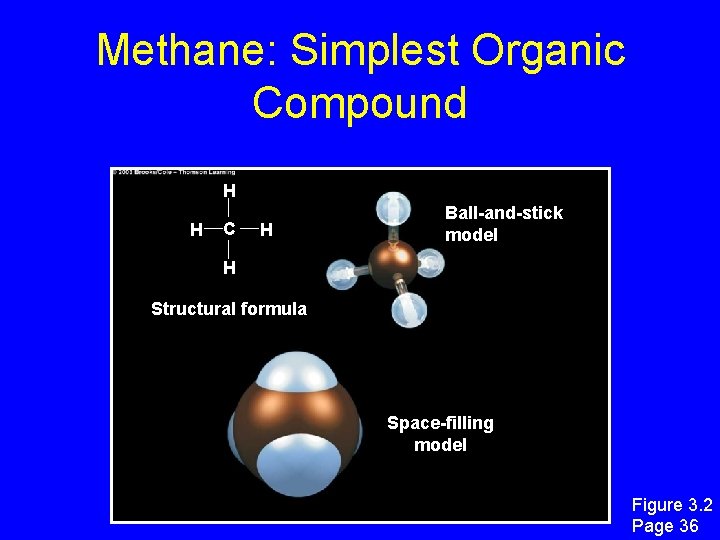
Methane: Simplest Organic Compound H H C H Ball-and-stick model H Structural formula Space-filling model Figure 3. 2 Page 36
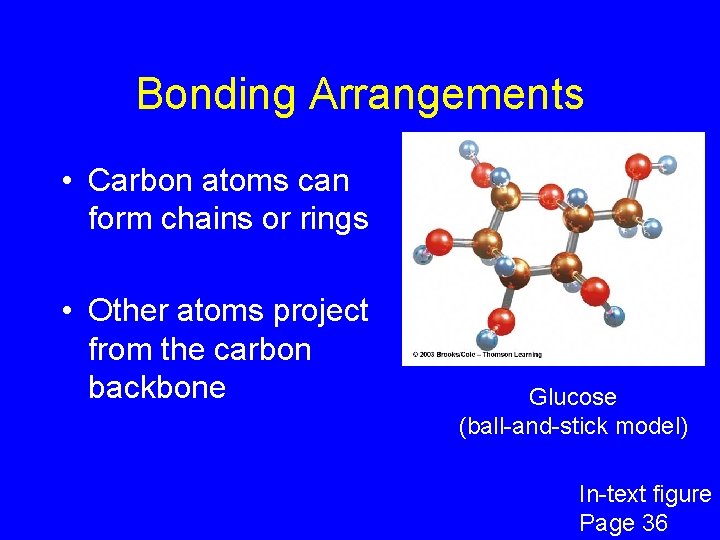
Bonding Arrangements • Carbon atoms can form chains or rings • Other atoms project from the carbon backbone Glucose (ball-and-stick model) In-text figure Page 36
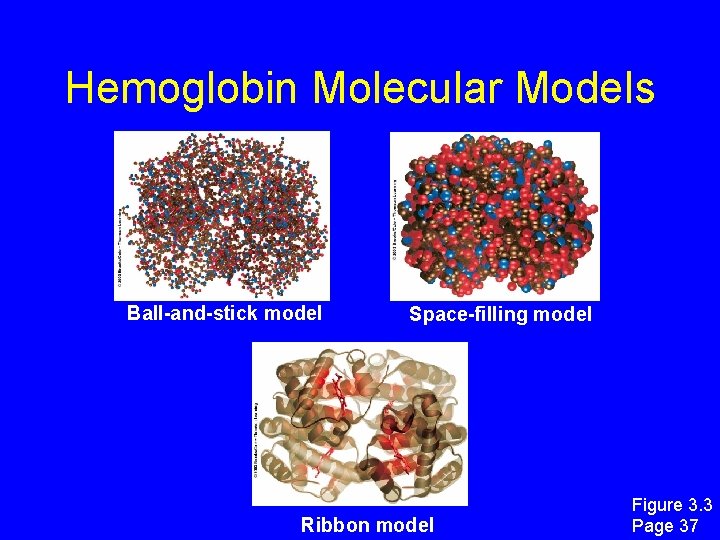
Hemoglobin Molecular Models Ball-and-stick model Space-filling model Ribbon model Figure 3. 3 Page 37
Functional Groups • Atoms or clusters of atoms that are covalently bonded to carbon backbone • Give organic compounds their different properties

Examples of Functional Groups Methyl group - CH 3 Hydroxyl group - OH Amino group - NH 3+ Carboxyl group - COOH Phosphate group - PO 3 - Sulfhydryl group - SH
Carbohydrates Monosaccharides (simple sugars) Oligosaccharides (short-chain carbohydrates) Polysaccharides (complex carbohydrates)

Lipids • Most include fatty acids – Fats – Phospholipids – Waxes • Sterols and their derivatives have no fatty acids • Tend to be insoluble in water
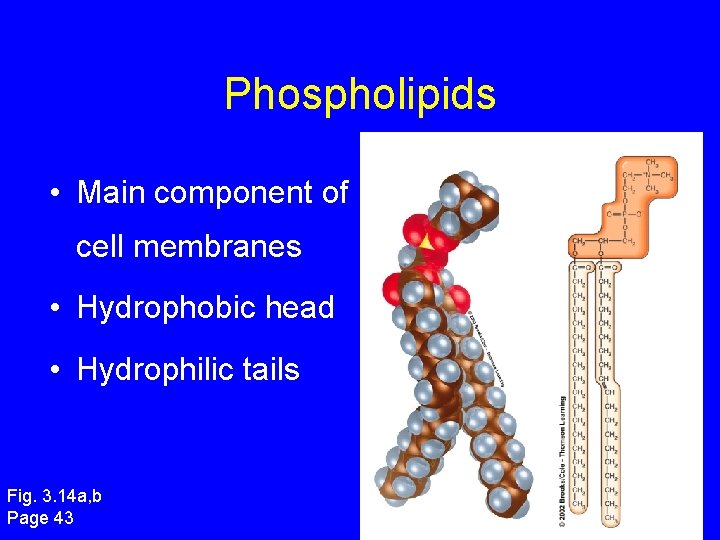
Phospholipids • Main component of cell membranes • Hydrophobic head • Hydrophilic tails Fig. 3. 14 a, b Page 43
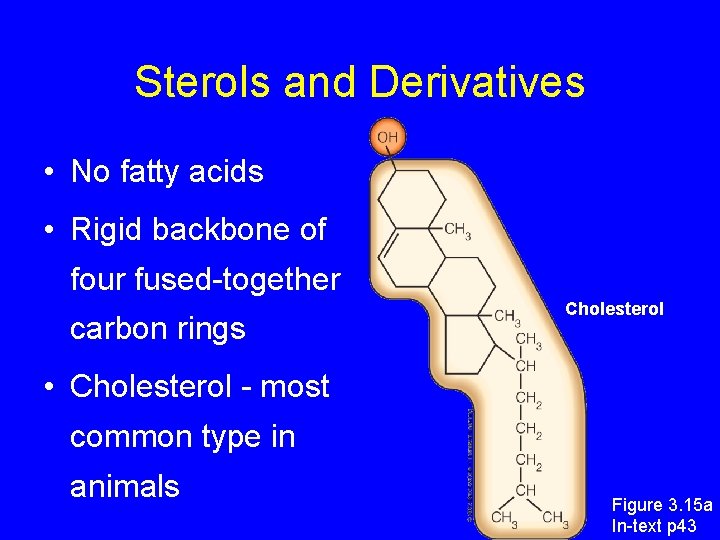
Sterols and Derivatives • No fatty acids • Rigid backbone of four fused-together carbon rings Cholesterol • Cholesterol - most common type in animals Figure 3. 15 a In-text p 43

Waxes • Long-chain fatty acids linked to long-chain alcohols or carbon rings • Firm consistency, repel water • Important in water-proofing
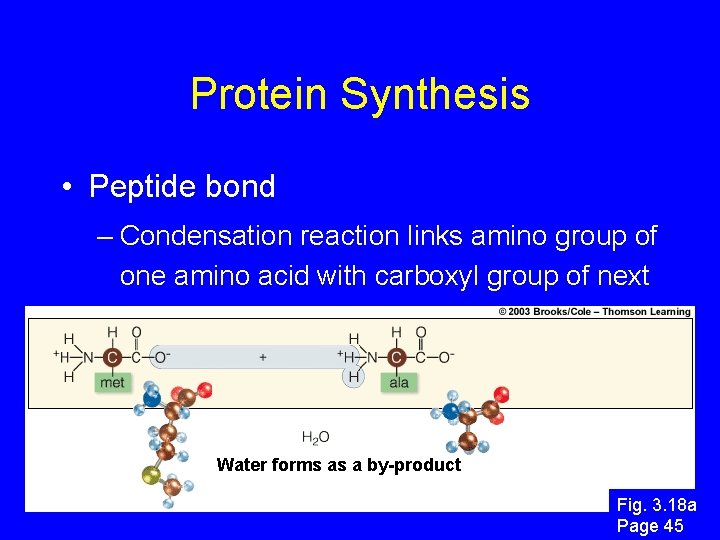
Protein Synthesis • Peptide bond – Condensation reaction links amino group of one amino acid with carboxyl group of next Water forms as a by-product Fig. 3. 18 a Page 45
Primary Structure • Sequence of amino acids • Unique for each protein • Two linked amino acids = dipeptide • Three or more = polypeptide • Backbone of polypeptide has N atoms: -N-C-C-N-C-C-N-

Second and Third Levels • Hydrogen bonding produces helix or sheet • Domain formation Tertiary structure Secondary structure Figure 3. 19 a Page 46
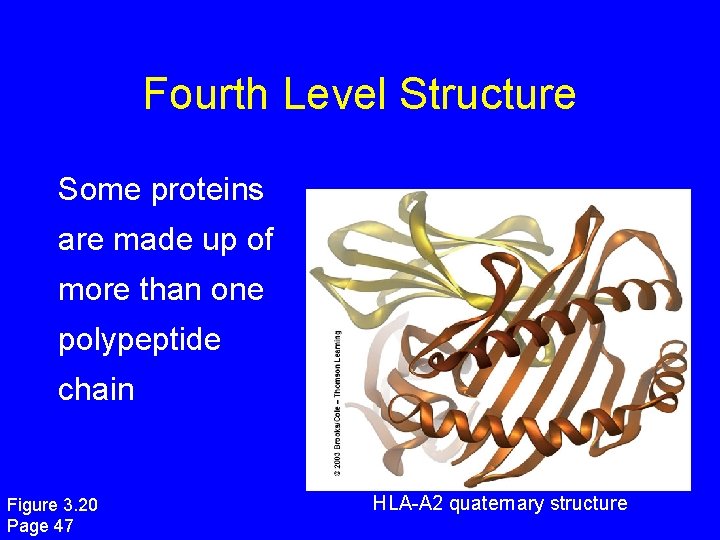
Fourth Level Structure Some proteins are made up of more than one polypeptide chain Figure 3. 20 Page 47 HLA-A 2 quaternary structure
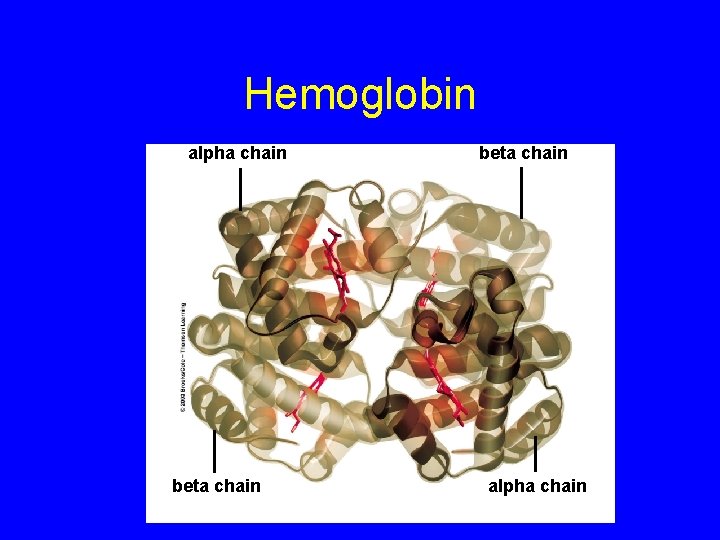
Hemoglobin alpha chain beta chain alpha chain
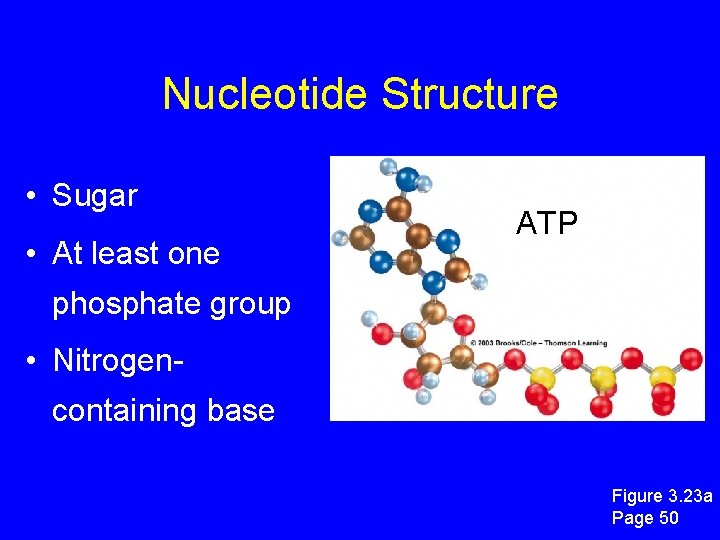
Nucleotide Structure • Sugar • At least one ATP phosphate group • Nitrogencontaining base Figure 3. 23 a Page 50
Nucleotide Functions • Energy carriers • Coenzymes • Chemical messengers • Building blocks for nucleic acids
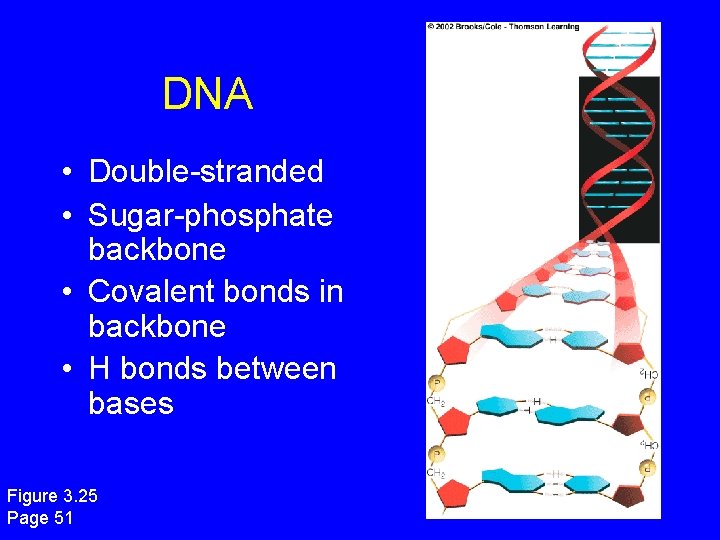
DNA • Double-stranded • Sugar-phosphate backbone • Covalent bonds in backbone • H bonds between bases Figure 3. 25 Page 51
RNA • Usually single strands • Four types of nucleotides • Unlike DNA, contains the base uracil in place of thymine • Three types are key players in protein synthesis
- Slides: 24