Carbon Compounds Biological Macromolecules Organic Compounds contain carbon

Carbon Compounds Biological Macromolecules

Organic Compounds • contain carbon atoms covalently bonded to other atoms – covalent bond = sharing electrons • has an entire branch of chemistry devoted to it called Organic Chemistry • makes up most matter in your body that is not water

Carbon Bonds Crash Course (You. Tube) Carbon is a Tramp (See Time 01: 46)
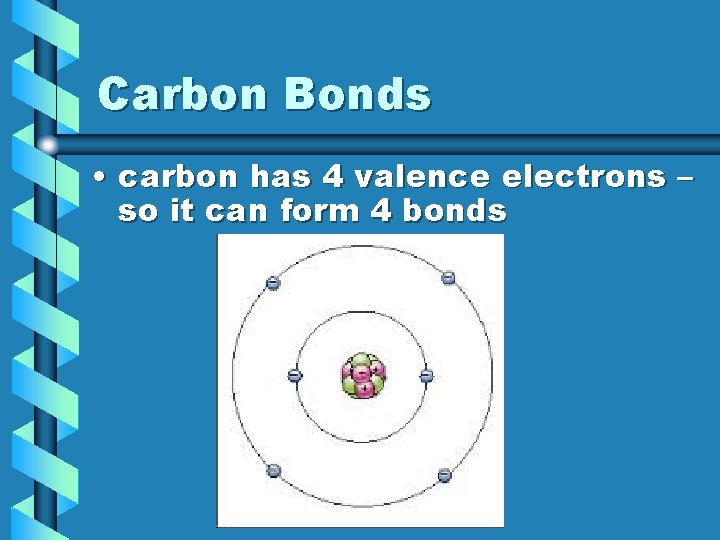
Carbon Bonds • carbon has 4 valence electrons – so it can form 4 bonds

Carbon Bonds • carbon has 4 valence electrons, allowing it to form 4 bonds – typically bonds with H, O, P, S, & N
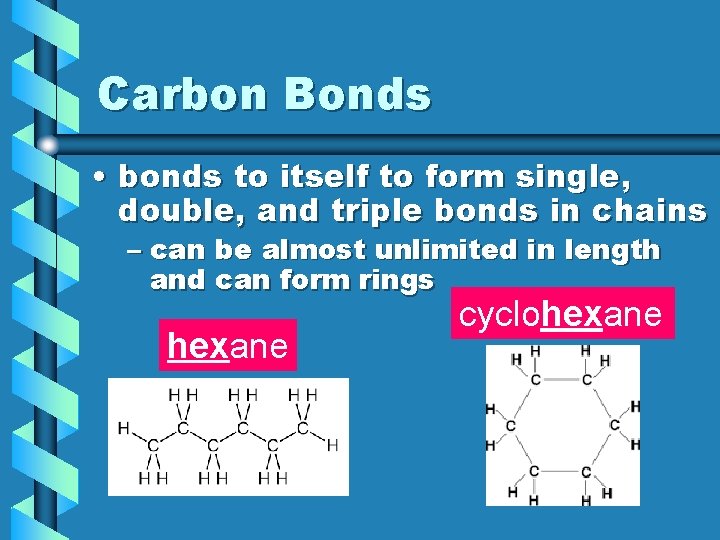
Carbon Bonds • bonds to itself to form single, double, and triple bonds in chains – can be almost unlimited in length and can form rings hexane cyclohexane

Macromolecules • a large molecule made from thousands or hundreds of thousands of smaller molecules
Monomers • single molecule able to bond in long chains
Polymers • many monomers joined together to form a macromolecule
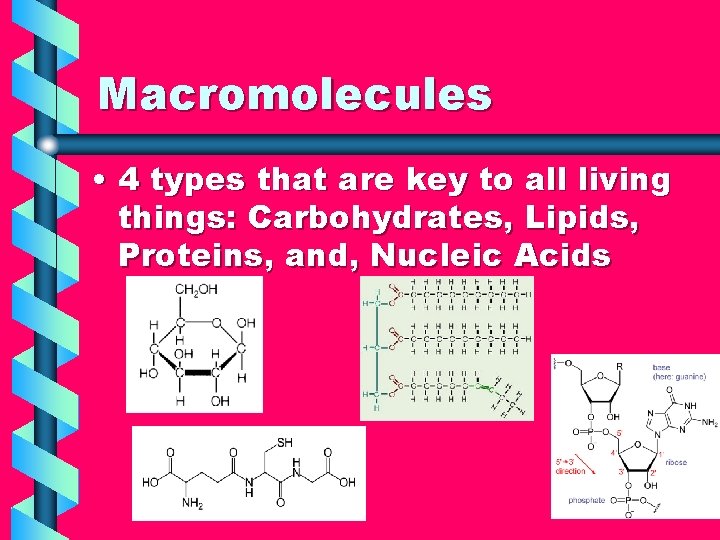
Macromolecules • 4 types that are key to all living things: Carbohydrates, Lipids, Proteins, and, Nucleic Acids
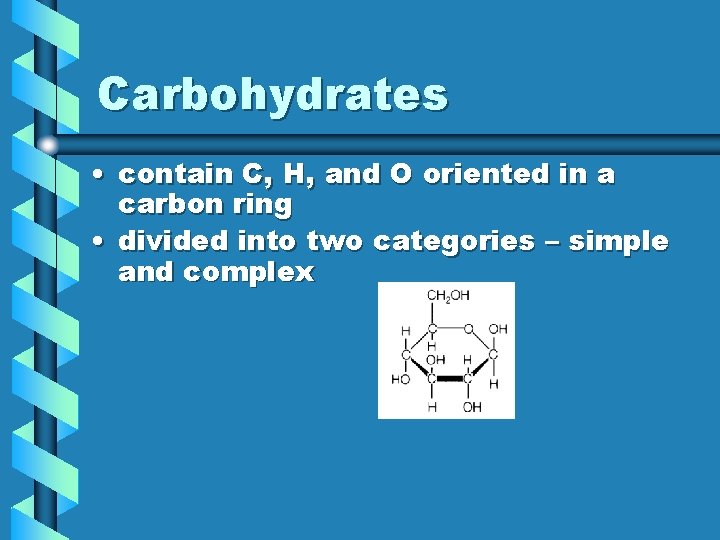
Carbohydrates • contain C, H, and O oriented in a carbon ring • divided into two categories – simple and complex

Carbohydrates • Crash. Course (You. Tube) – Carbohydrates (see Time 03: 42)
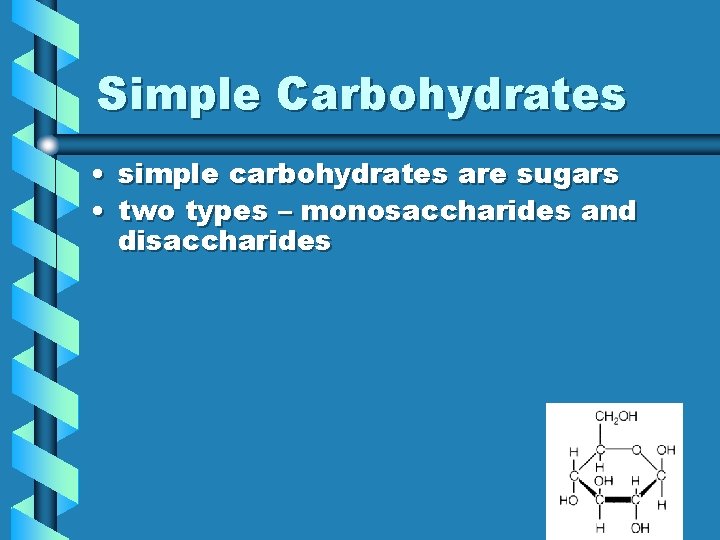
Simple Carbohydrates • simple carbohydrates are sugars • two types – monosaccharides and disaccharides
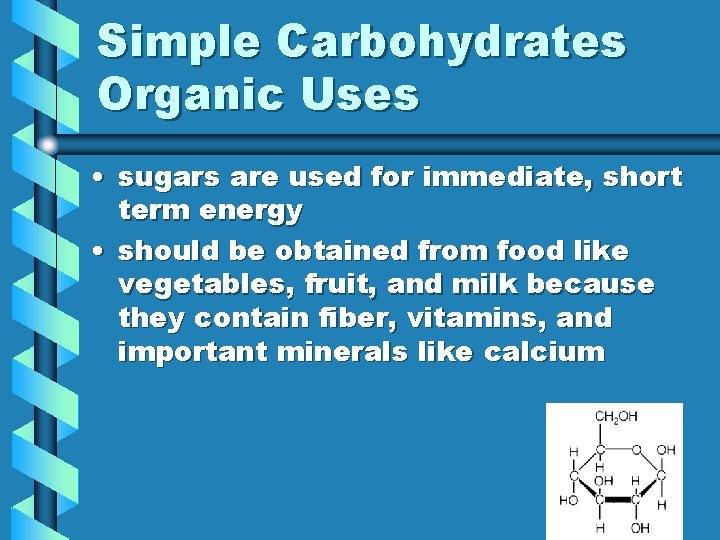
Simple Carbohydrates Organic Uses • sugars are used for immediate, short term energy • should be obtained from food like vegetables, fruit, and milk because they contain fiber, vitamins, and important minerals like calcium

Carbohydrate Monomer • Monosaccharide = one sugar = made of 1 Ring – many monosaccharides can be bonded together – glucose (juice, sweets), fructose (fruit), and galactose

Two Part Sugars • Disaccharide = 2 sugars = made of 2 rings bonded together – formed when two monosaccharides bond together – maltose (malts), sucrose (table sugar), and lactose (dairy)
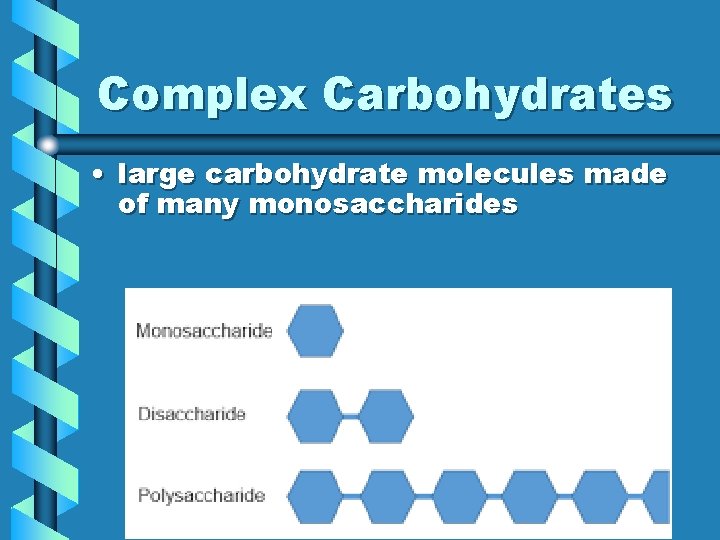
Complex Carbohydrates • large carbohydrate molecules made of many monosaccharides
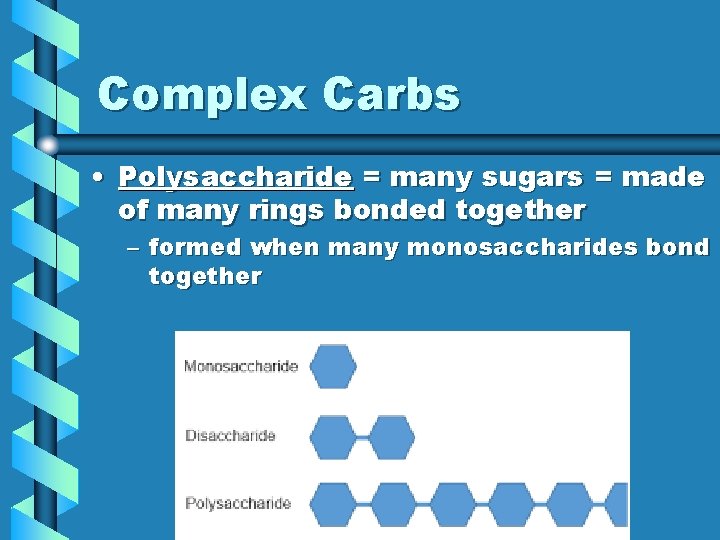
Complex Carbs • Polysaccharide = many sugars = made of many rings bonded together – formed when many monosaccharides bond together
Organic Uses of Complex Carbs • many different functions based on structure – long term energy – build some biological structures – Ex: plant parts – provide a small amount of energy storage
Different Complex Carbs and Their Organic Uses • Starch – complex carbohydrate made by plants for energy storage – provides humans with long term energy when eaten – should be eaten the MOST of any food for energy

Different Complex Carbs and Their Organic Uses • Cellulose – complex carbohydrate made by plants to build cell walls

Different Complex Carbs and Their Organic Uses • Fiber – group of many types of complex carbohydrates (including cellulose) also known as roughage – indigestible form of many carbohydrates – help clean out our digestive system
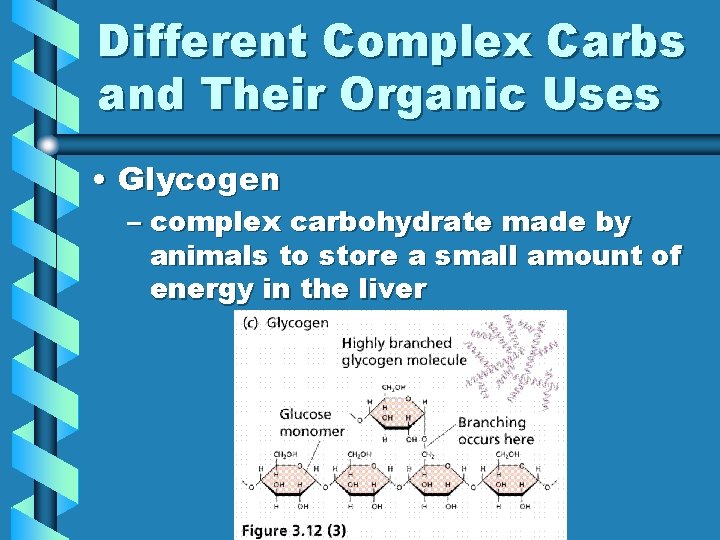
Different Complex Carbs and Their Organic Uses • Glycogen – complex carbohydrate made by animals to store a small amount of energy in the liver
Lipids • large C-H molecules with some O that are not soluble in water – lipids are nonpolar • should eat less lipids than carbohydrates – very calorie-dense
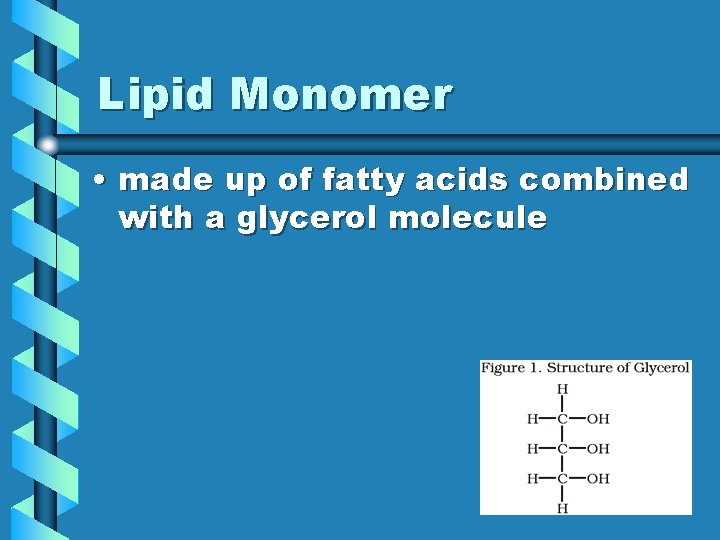
Lipid Monomer • made up of fatty acids combined with a glycerol molecule

Lipids • Crash. Course (You. Tube) – Lipids (See Time 07: 02)

Organic Uses of Lipids • long term energy storage • producing waterproof structures like cell membranes and wax • hormones (ex: estrogen) to send chemical messages in blood • absorbing some vitamins

Common Types of Lipids • Fats • Oils • Steroids • Waxes
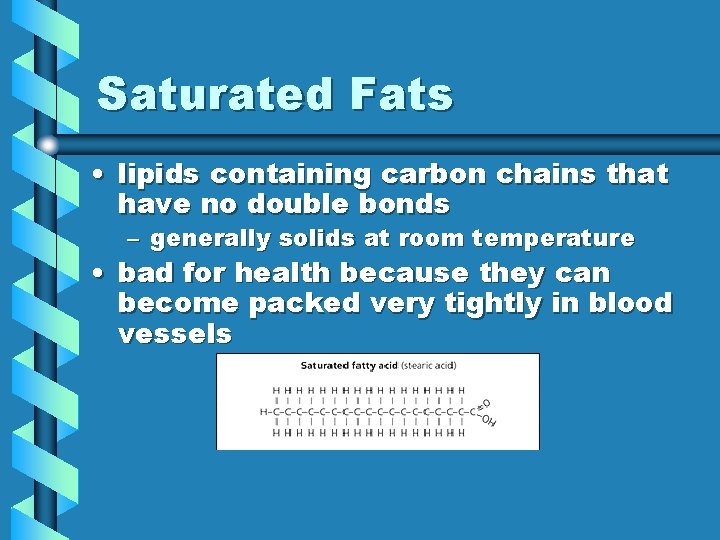
Saturated Fats • lipids containing carbon chains that have no double bonds – generally solids at room temperature • bad for health because they can become packed very tightly in blood vessels
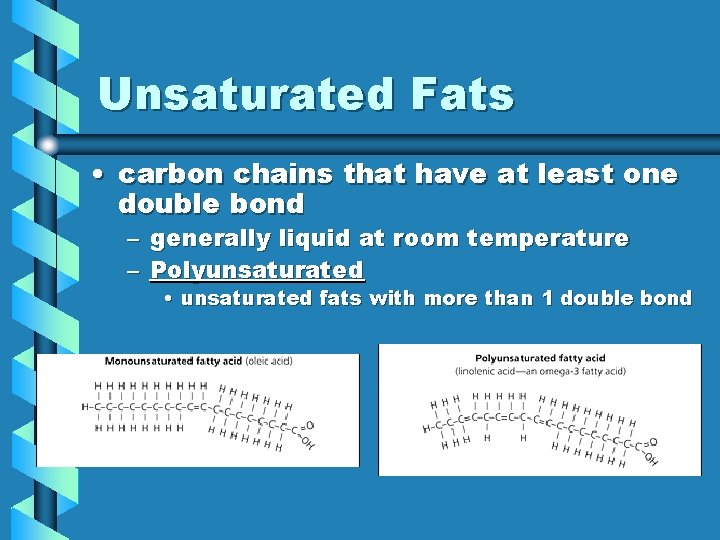
Unsaturated Fats • carbon chains that have at least one double bond – generally liquid at room temperature – Polyunsaturated • unsaturated fats with more than 1 double bond
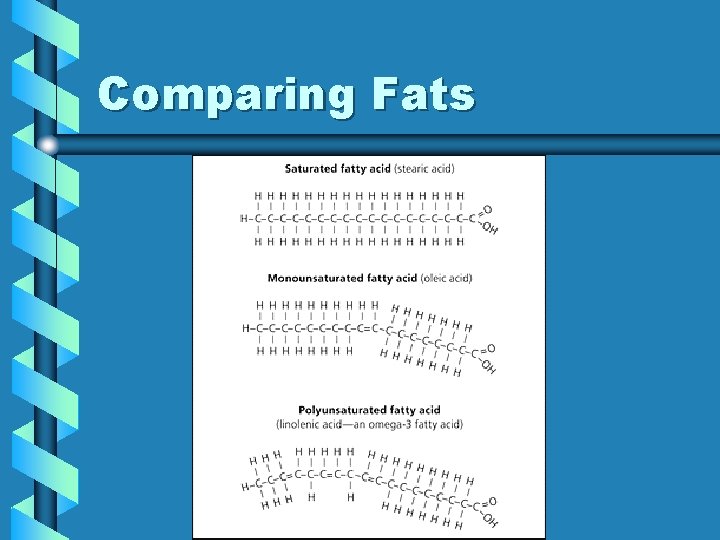
Comparing Fats

Trans Fats

Trans Fats • naturally unsaturated fatty acids that have been saturated artificially by the addition of hydrogen atoms

Trans Fats • used to extend shelf life of food and to enhance food flavor, etc. , but at a dangerous price………. – coronary heart disease – liver dysfunction
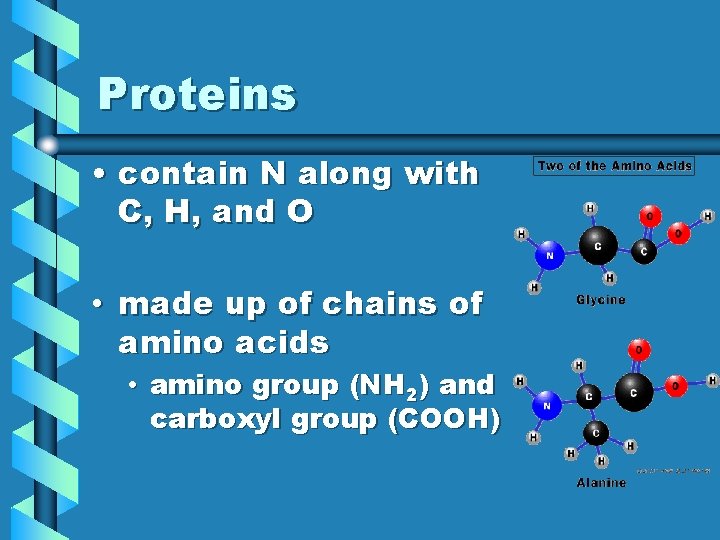
Proteins • contain N along with C, H, and O • made up of chains of amino acids • amino group (NH 2) and carboxyl group (COOH)
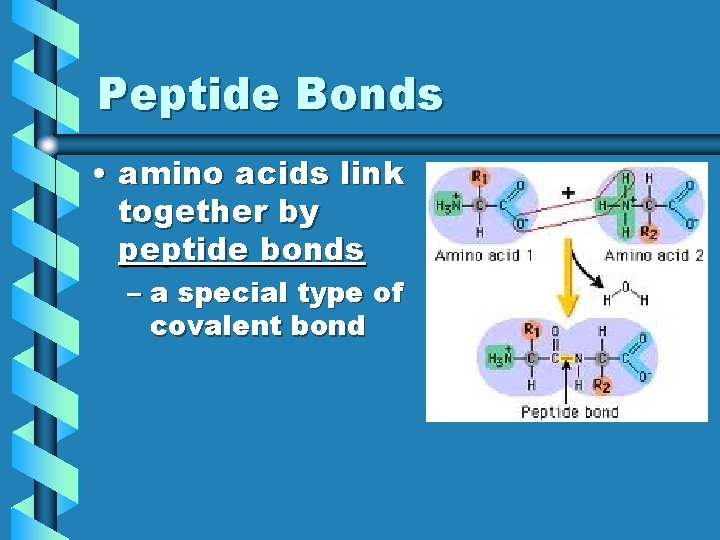
Peptide Bonds • amino acids link together by peptide bonds – a special type of covalent bond

Proteins • Crash. Course (You. Tube) – Proteins (See Time 10: 42)
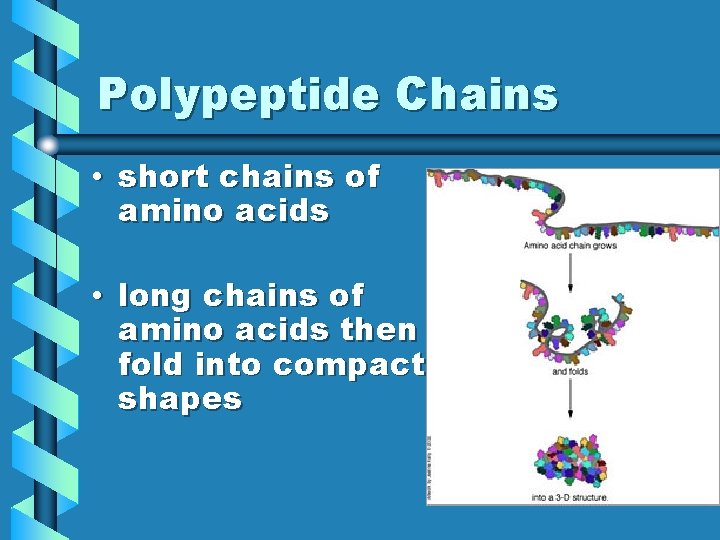
Polypeptide Chains • short chains of amino acids • long chains of amino acids then fold into compact shapes

How many types of amino acids are found in nature? • 20 different amino acids found in nature

Amino Acids • Nonessential amino acids – can be made by our bodies • Essential amino acids – we must eat them to get them

Proteins in our bodies • proteins make up the second biggest portion of our body weight • we should eat proteins less than carbohydrates • our bodies can make many amino acids

Proteins in our bodies • genes (DNA) code for how to make proteins – proteins determine what we look like and how our bodies work

Organic Uses of Proteins • muscles • skin and fingernails • hormones (ex: insulin) • enzymes (which speed up chemical reactions)
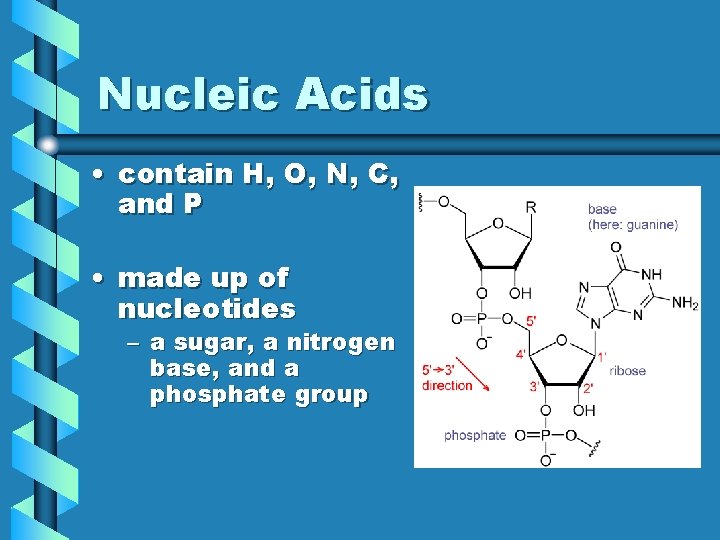
Nucleic Acids • contain H, O, N, C, and P • made up of nucleotides – a sugar, a nitrogen base, and a phosphate group
Nucleic Acids • Two Types of Nucleic Acids are – DNA (Deoxyribonucleic acid) – RNA (Ribonucleic acid)
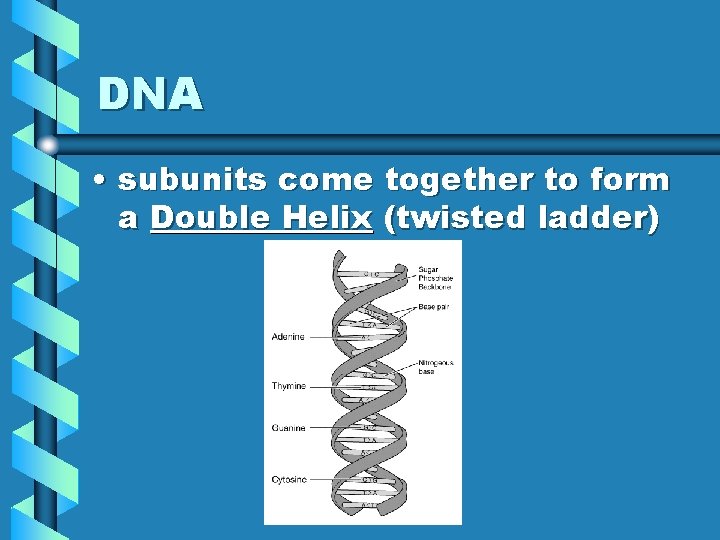
DNA • subunits come together to form a Double Helix (twisted ladder)

DNA • stores genetic information and codes for making your bodies’ proteins

RNA • a copy of DNA that carries a message (code) through the cell • the code is then used to build proteins

Summary • Carbohydrates, lipids, proteins, and nucleic acids are all polymers. • Why is a polymer like a chain?
- Slides: 49