Carbon Compounds BIOCHEMISTRY I Role of Carbon in

Carbon Compounds BIOCHEMISTRY

I. Role of Carbon in Organisms Organic compounds = compounds __________ that contain carbon Ex: carbohydrates, lipids, proteins Inorganic compounds = compounds ___________ that DO NOT contain carbon Ex: vitamins, minerals, water
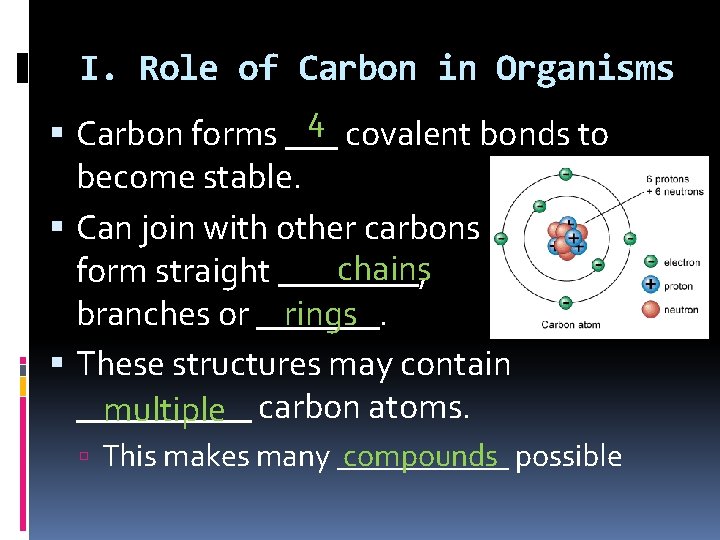
I. Role of Carbon in Organisms 4 Carbon forms ___ covalent bonds to become stable. Can join with other carbons chains form straight ____, branches or _______. rings These structures may contain _____ multiple carbon atoms. compounds possible This makes many ______ to
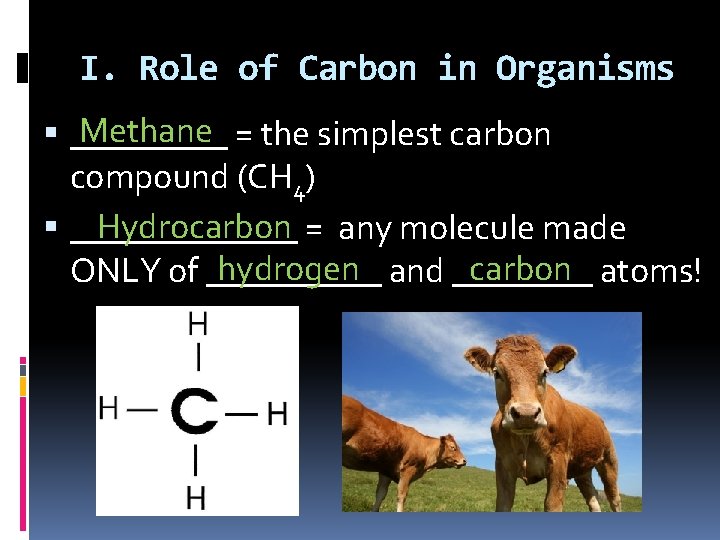
I. Role of Carbon in Organisms Methane = the simplest carbon _____ compound (CH 4) Hydrocarbon = any molecule made _______ hydrogen and ____ carbon atoms! ONLY of _____
II. The Digestive System The digestive system breaks down organic compounds into their building blocks (_____). monomers Body cells take the monomers and put them together in the form the body can use

II. The Digestive System Macromolecules = extremely large ________ compounds made of smaller compounds. _____ Polymer = large molecule formed when many smaller molecules (monomers) long chains. bond together, usually in ______ Ex: carbohydrates, proteins, lipids, nucleic acids
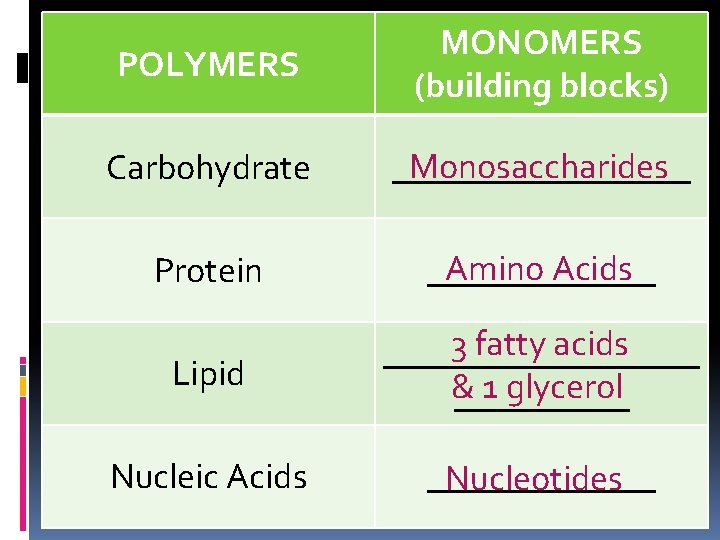
POLYMERS MONOMERS (building blocks) Carbohydrate Monosaccharides _________ Protein Amino Acids _______ Lipid 3 fatty acids _________ & 1 glycerol _____ Nucleic Acids _______ Nucleotides

What do athletes eat the day before a big game? Carbohydrates: Carb loading works because carbohydrates are used by the cells to STORE and RELEASE energy.

III. Carbohydrates storage and Compounds used for _____ energy release of ____ Made of C, H, O atoms
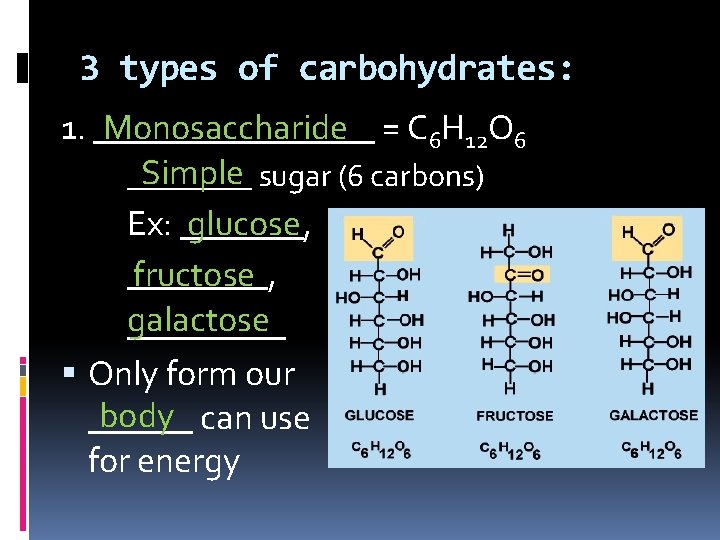
3 types of carbohydrates: Monosaccharide = C 6 H 12 O 6 1. ________ Simple sugar (6 carbons) ____ Ex: _______, glucose ____, fructose galactose _____ Only form our body can use ______ for energy

3 types of carbohydrates: Disaccharide 2. ________ = C 12 H 22 O 11 Double sugar made of 2 simple sugars ____ lactose (milk sugar), _____, maltose Ex: _____ sucrose (table sugar) _____

3 types of carbohydrates: Polysaccharide = 3. ________ monosaccharides more than 2 _________ Ex. Starch - plant’s energy storing molecule ____
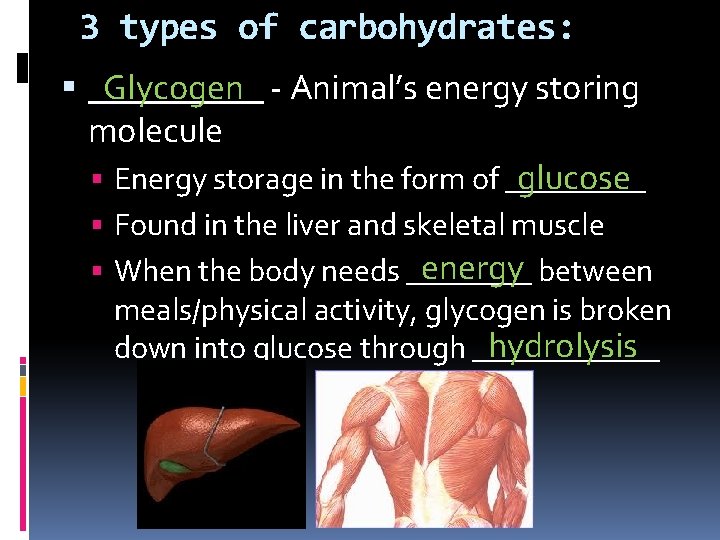
3 types of carbohydrates: _____ Glycogen - Animal’s energy storing molecule glucose Energy storage in the form of _____ Found in the liver and skeletal muscle energy between When the body needs ____ meals/physical activity, glycogen is broken hydrolysis down into glucose through ______
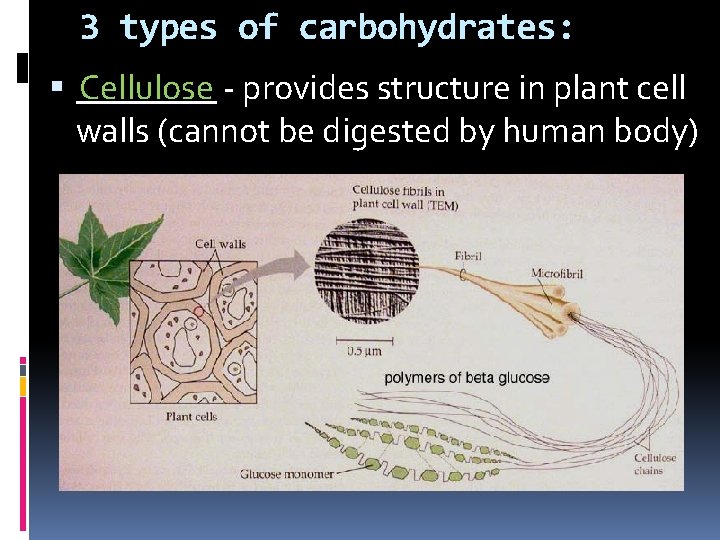
3 types of carbohydrates: ____ Cellulose - provides structure in plant cell walls (cannot be digested by human body)
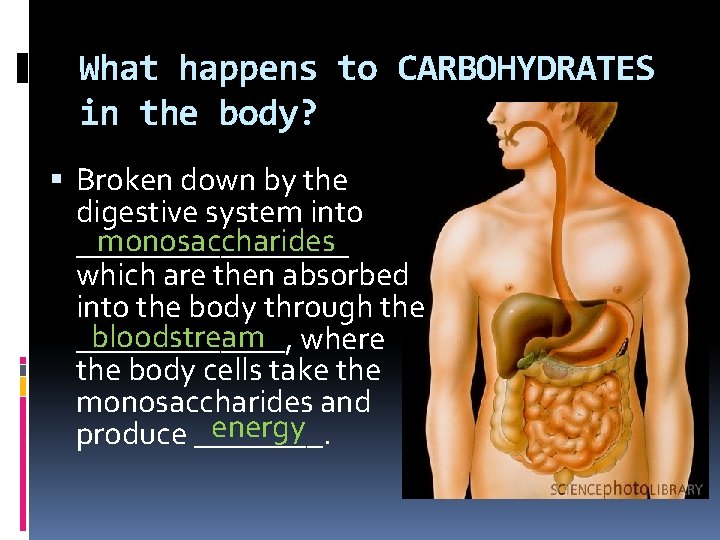
What happens to CARBOHYDRATES in the body? Broken down by the digestive system into monosaccharides _________ which are then absorbed into the body through the bloodstream where _______, the body cells take the monosaccharides and energy produce ____.

Lipids & Proteins BIOCHEMISTRY

I. Lipids fats and _______ oils Commonly called _______ Contain ______ less more C-H bonds and ______ carbohydrates O atoms than ________ Ex. C 57 H 110 O 6 Nonpolar; therefore water repel _______ insoluble (_____)

I. Lipids Functions of lipids in your body ______________: 1. ______ Long term energy storage (used when carbohydrates are _____ NOT available) Insulation 2. _____ 3. _____ Protect body tissue (cushioning)
Which has more energy lipids or carbs? TWICE as One gram of _____ fat contains _______ energy as one gram of much _______________. carbohydrates fats Therefore, _____ are better _______ storage compounds!
Fats vs. Carbs & Energy Storage 1 gram of Carbs (glycogen) = 4 Kcal of energy about ___ A short term rapid energy source (sprint events) 1 gram of Fats = about 9 Kcal of energy _______ A long term energy source (endurance events – marathons)
Types and Examples of Lipids: Sterols - steroids 1. _______ Waxes - bee, furniture, ear 2. ______ Cholesterol - in egg yolks 3. _____ Fats - from animals 4. _____ Oils - from plants 5. ____

Structure of Lipids Basic building blocks: 3 fatty acids + ______ 1 glycerol ________ Fatty Acids _______ Long ________ carboxyl chains of carbon with a _____ group at one end.
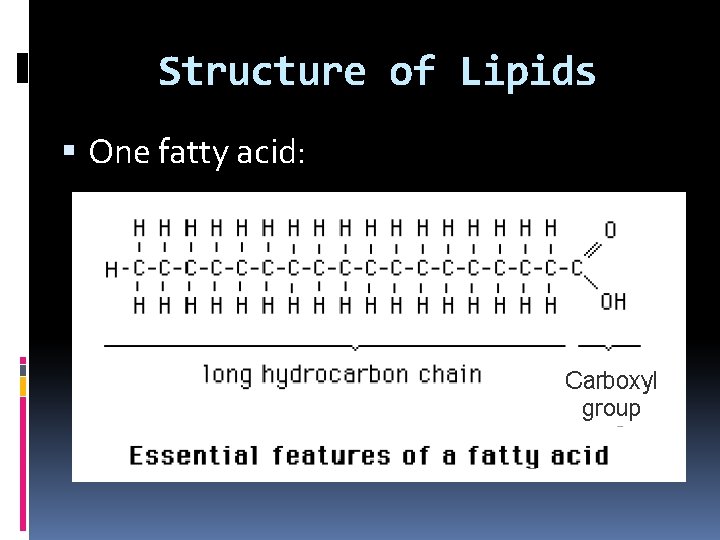
Structure of Lipids One fatty acid:
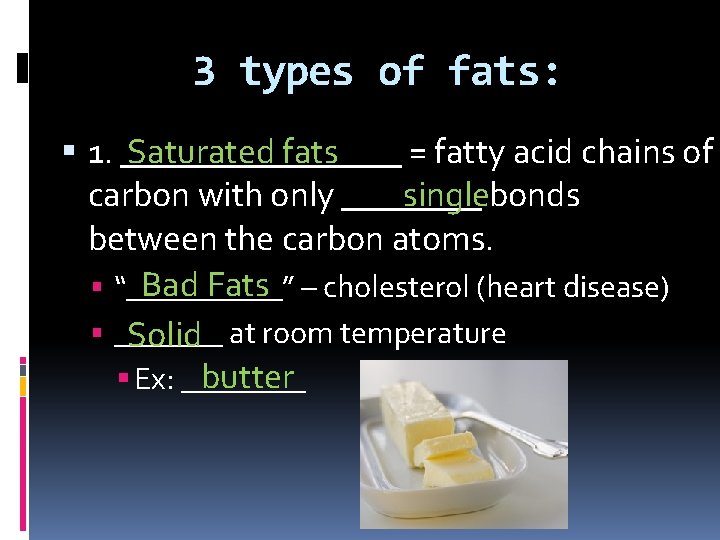
3 types of fats: 1. ________ Saturated fats = fatty acid chains of singlebonds carbon with only ____ between the carbon atoms. Bad Fats – cholesterol (heart disease) “_____” _______ Solid at room temperature butter Ex: ____

3 types of fats: 2. _________ Unsaturated fats = fatty acid chains double of carbon with ONE ____ bond between the carbon atoms Good Fats “______” ____ liquid at room temperature olive oil Ex: ______

3 types of fats: 3. ___________ Polyunsaturated fats = more than one double bond between the carbon atoms in the chain. Ex: nuts, seeds, fish, leafy greens.
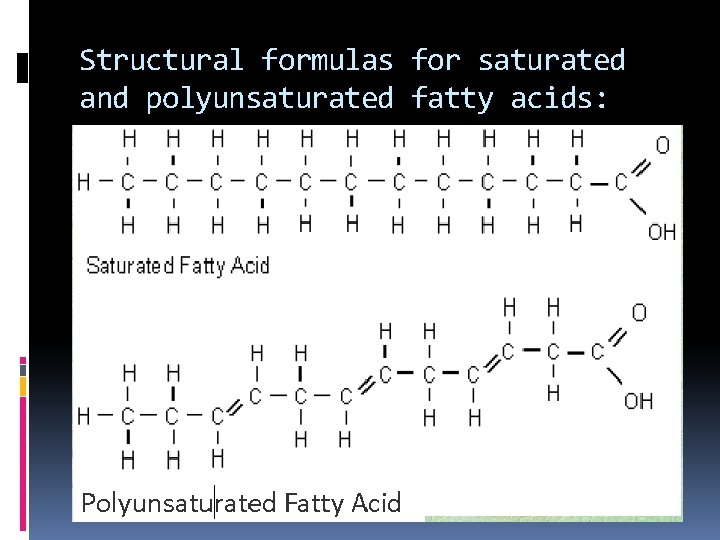
Structural formulas for saturated and polyunsaturated fatty acids:
What happens to LIPIDS in the body? Broken down by the digestive system into fatty acids and glycerol ___________ which are then absorbed into the body through the bloodstream, where the body cells take the fatty acids and glycerol and make needed lipids.
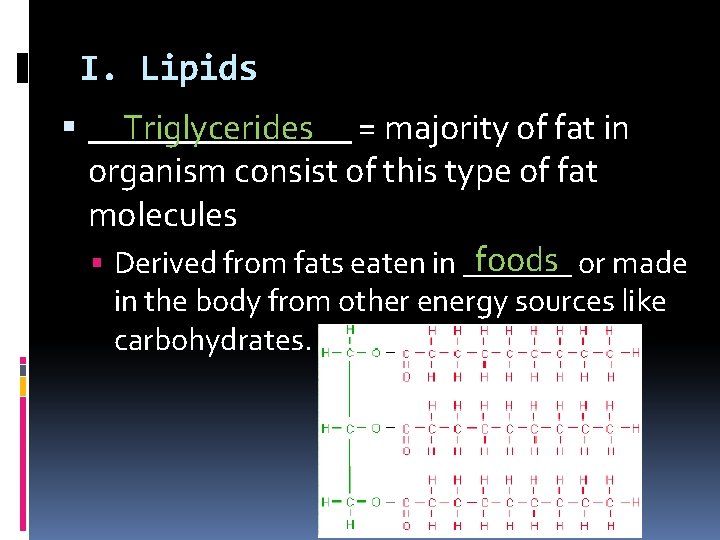
I. Lipids Triglycerides = majority of fat in ________ organism consist of this type of fat molecules foods or made Derived from fats eaten in _______ in the body from other energy sources like carbohydrates.

I. Lipids Calories ingested in a meal and not used immediately by tissues are converted to triglycerides and transported to fat cells to be stored. energy vs. Storage – 3 month supply of ____ glycogen’s 24 hour supply.
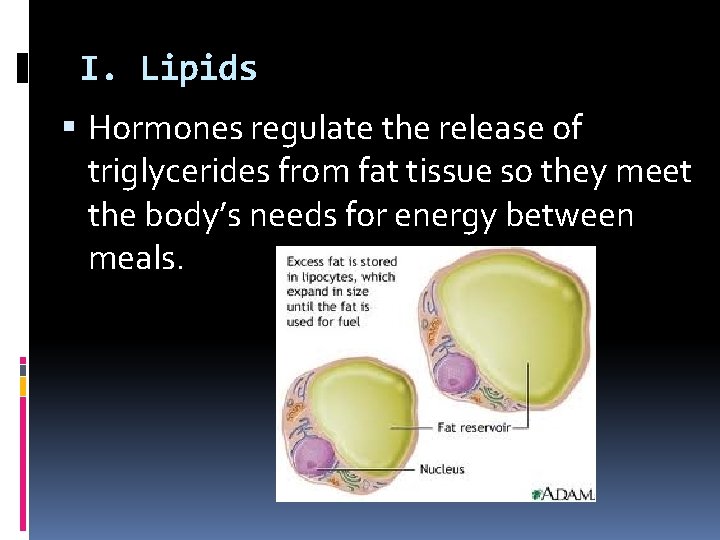
I. Lipids Hormones regulate the release of triglycerides from fat tissue so they meet the body’s needs for energy between meals.
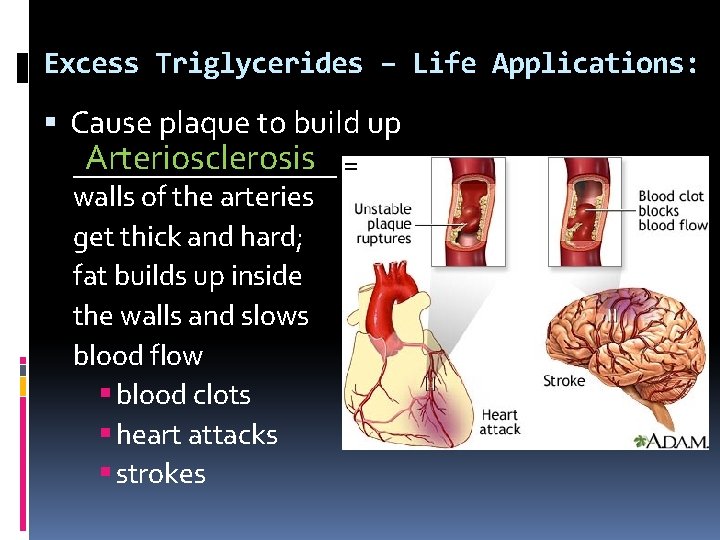
Excess Triglycerides – Life Applications: Cause plaque to build up Arteriosclerosis = _________ walls of the arteries get thick and hard; fat builds up inside the walls and slows blood flow blood clots heart attacks strokes
Excess Triglycerides – Life Applications: Hypertension ______ high blood pressure
II. Proteins Large complex polymer composed of C, H, O, N and sometimes S Monomers (basic building blocks): amino acids _______ 20 different ___ amino acids
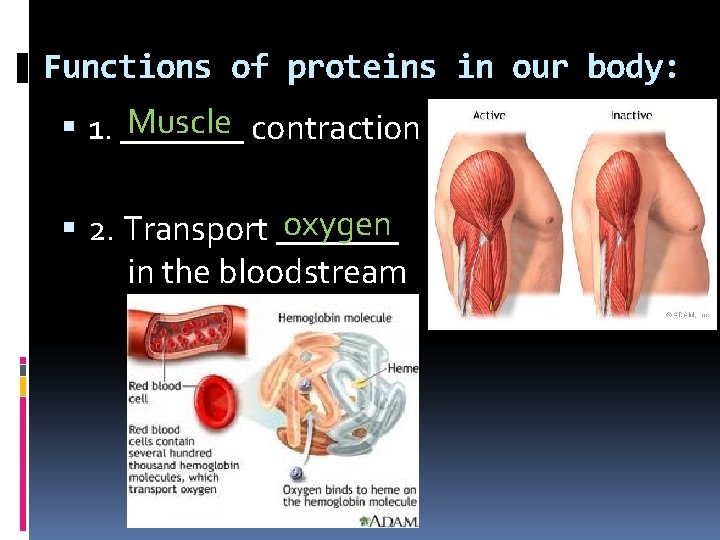
Functions of proteins in our body: Muscle contraction 1. _______ oxygen 2. Transport _______ in the bloodstream
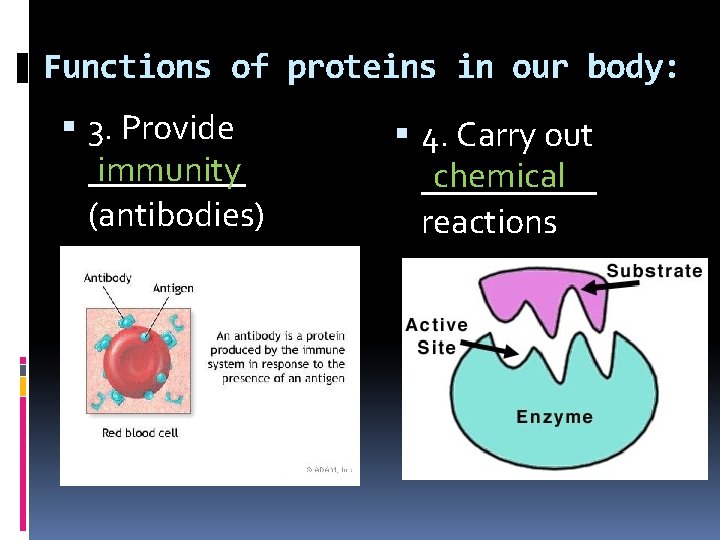
Functions of proteins in our body: 3. Provide immunity _____ (antibodies) 4. Carry out chemical _____ reactions
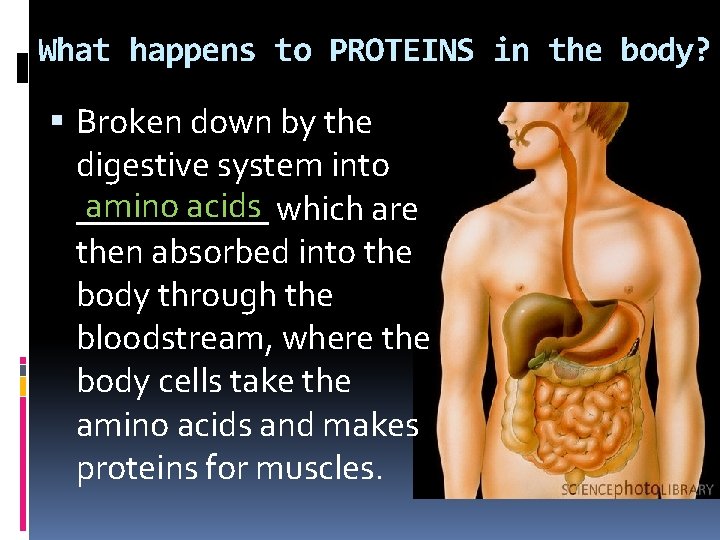
What happens to PROTEINS in the body? Broken down by the digestive system into amino acids which are ______ then absorbed into the body through the bloodstream, where the body cells take the amino acids and makes proteins for muscles.
Enzymes & Nucleic Acids BIOCHEMISTRY

I. Enzymes: protein A specialized type of _____ Function in your body ____________: acts catalyst = substance that likes a _____ speeds up the rate of a chemical ____ reaction but it is NOT ____ used up in the reaction.

I. Enzymes: activation Enzyme(s) reduce ______ energy = amount of energy needed ____ to begin a reaction. Coenzyme = an organic molecule ____________ associated with the enzyme to help in the reaction ______
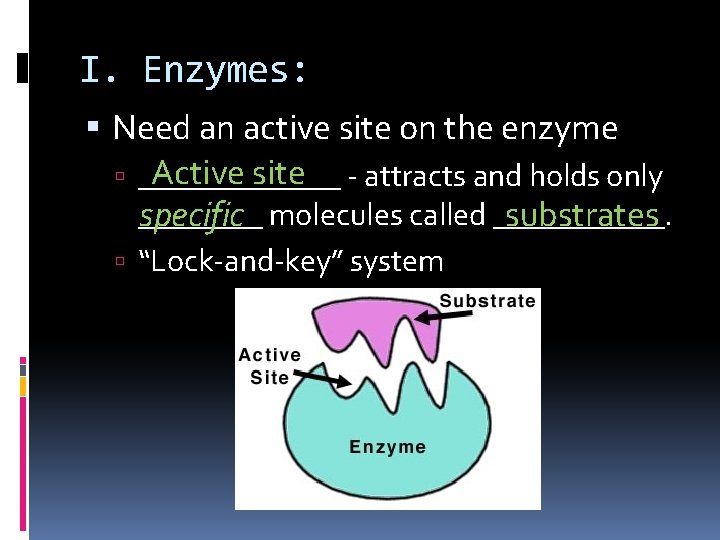
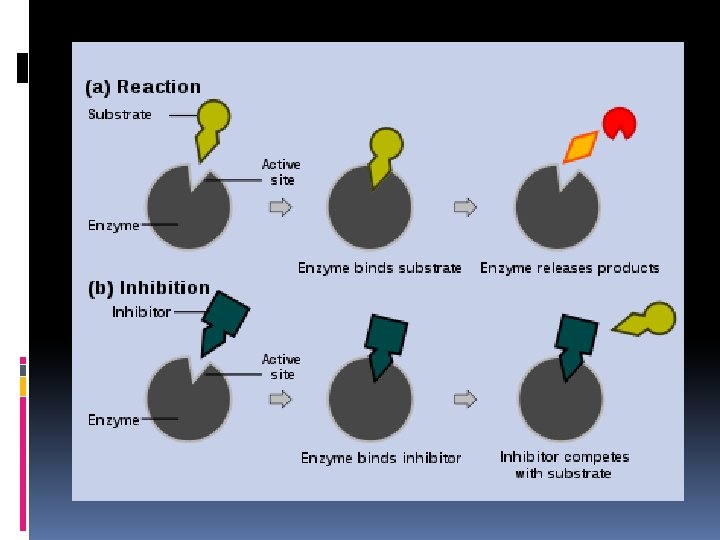
I. Enzymes: Need an active site on the enzyme Active site - attracts and holds only _______ specific molecules called ______. substrates “Lock-and-key” system
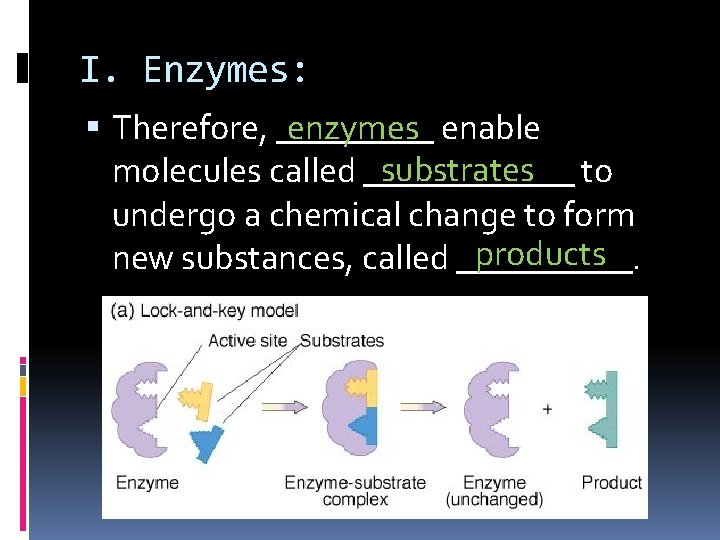
I. Enzymes: enzymes enable Therefore, _____ substrates to molecules called ______ undergo a chemical change to form products new substances, called _____.
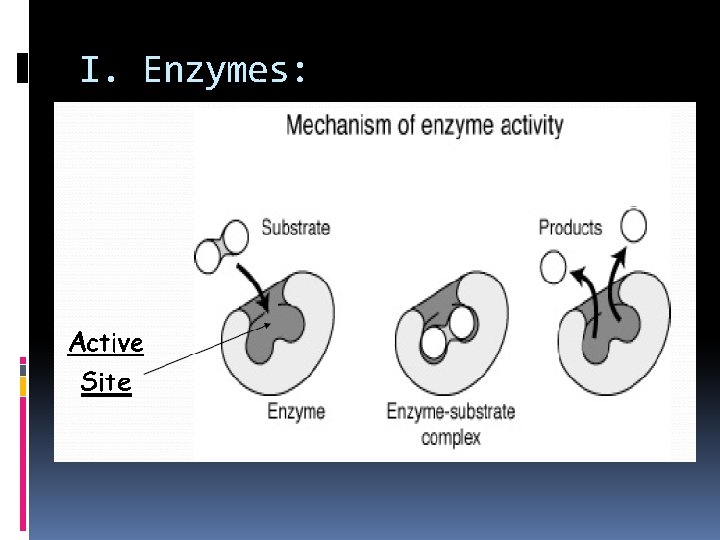
I. Enzymes:
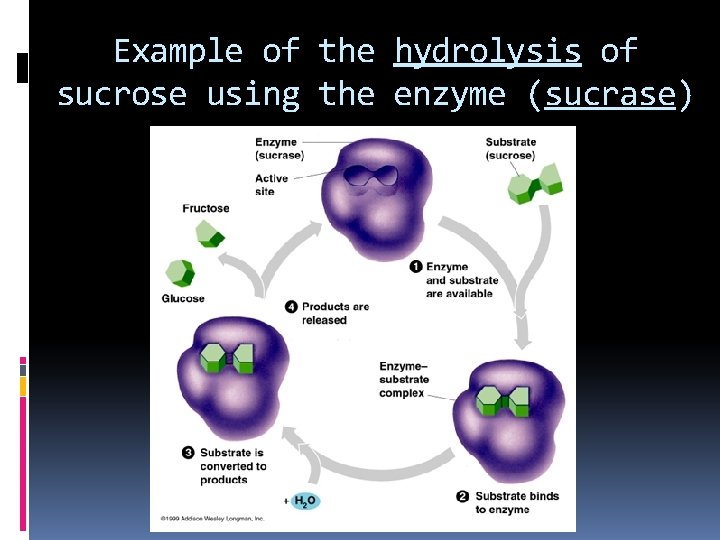
Example of the hydrolysis of sucrose using the enzyme (sucrase)

I. Enzymes: Competitive Inhibitors ____________ =a substance that _____ reduces the activity active of an enzyme by entering the _______ substrate site in place of the ______ mimics whose structure it ____.
I. Enzymes: survive without enzymes! Could not _____ (almost all chemical reactions in cells require an enzyme) Speed up the reactions in ______________: � 1. ______ Digestion of food Synthesis of molecules � 2. ______ Storage and release of energy � 3. __________

I. Enzymes: Enzymes are named for the _____ compound they work on. You drop the current compound ending and replace it with ______. -ase

I. Enzymes: For example: Lactose’s enzyme is _____ lactase maltase Maltose’s enzyme is _____ sucrase Sucrose’s enzyme is _________ Amylase (in your saliva) is the enzyme for starch
I. Enzymes: 2 Factors that affect enzymes _______________: - too high or too low will denature (break apart) enzymes 1. _______ Temperature p. H 2. ___

How does our body get energy from the breaking down of molecules? _____________ Energy is released when a chemical bond is broken ____________!
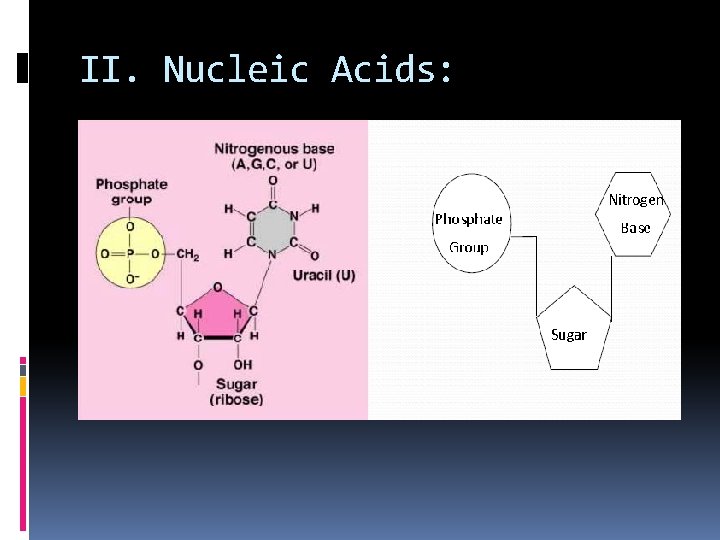
II. Nucleic Acids: Complex polymer that stores information code in cells in the form of a ______. Monomers (basic building blocks): _____, nucleotides which consist of C, H, O, N, P These elements are arranged in 3 groups: nitrogen base _______, simple sugar and a ________, _________. phosphate group
II. Nucleic Acids:
II. Nucleic Acids: DNA (deoxyribonucleic acid) 1. _____ contains all the instructions for organisms development. . . AKA genetic information 2. _____ a RNA (ribonucleic acid) forms copy of DNA and is used for _____ protein (production) synthesis
- Slides: 55