Carbon Compounds 6 3 Pages 157 163 Carbon

Carbon Compounds 6. 3 Pages 157 -163
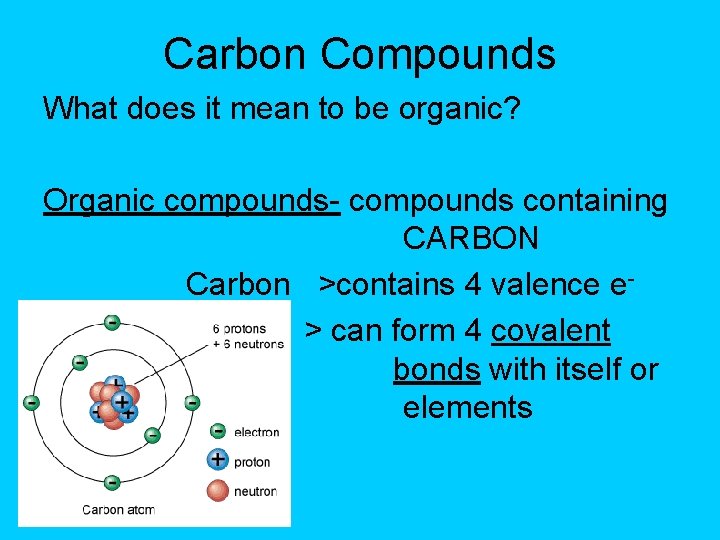
Carbon Compounds What does it mean to be organic? Organic compounds- compounds containing CARBON Carbon >contains 4 valence e> can form 4 covalent bonds with itself or other elements
Large carbon compounds (analogy= necklace) • Monomers are simple carbon molecules. Ex. Link in necklace • Polymers are molecules made of many monomers. (monomer + monomer = polymer) Ex. Whole necklace • Macromolecules are made of many polymers (polymer + polymer = macromolecule) Ex. Multi chain necklace
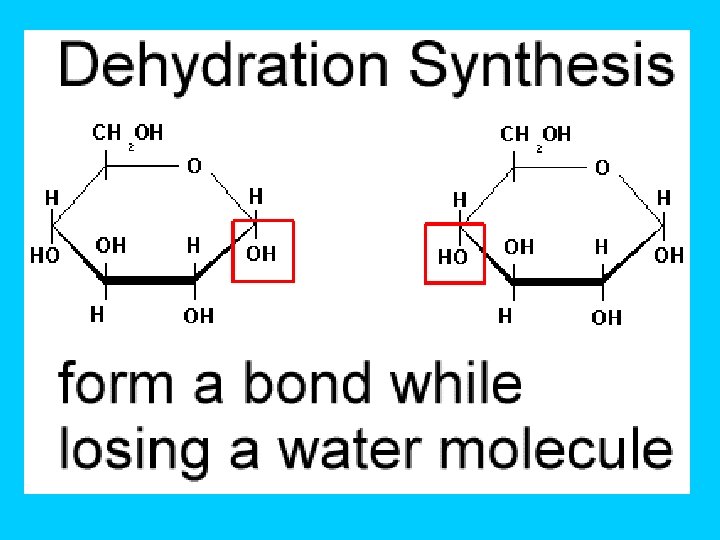
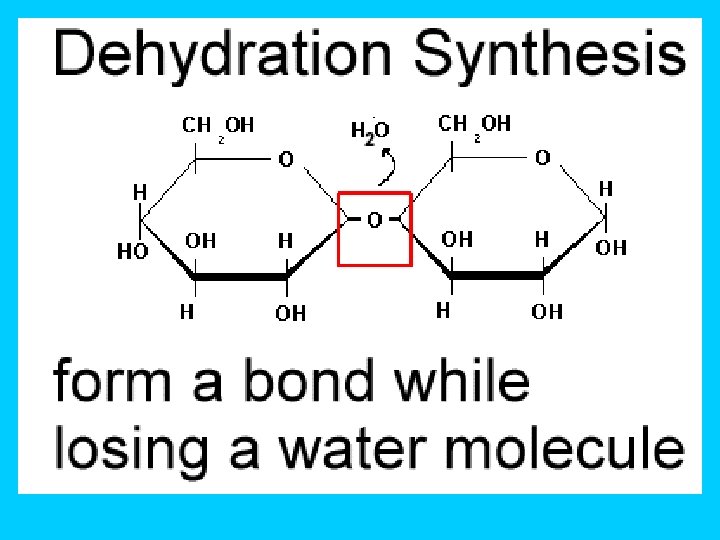
How do Monomers link to form Polymers? ? ? …through condensation reactions (called dehydration synthesis) Dehydration synthesis- chemical reaction in which one monomer donates a hydroxyl (OH-) and the other monomer donates a hydrogen (H) forming water (H 2 O)
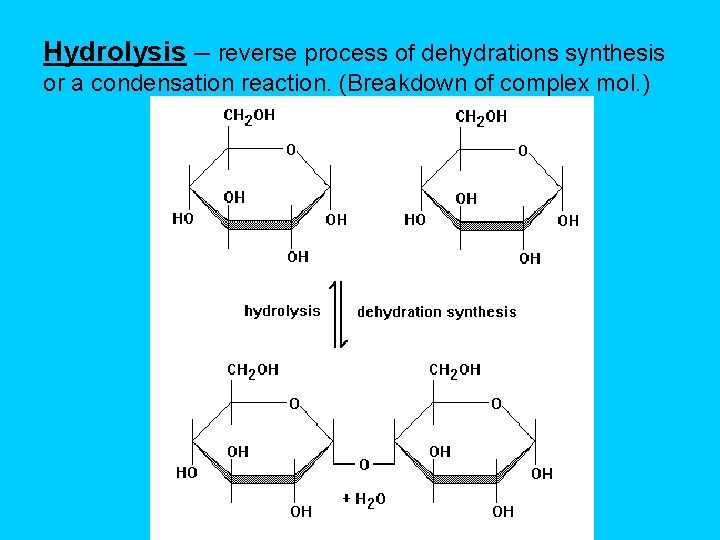
Hydrolysis – reverse process of dehydrations synthesis or a condensation reaction. (Breakdown of complex mol. )
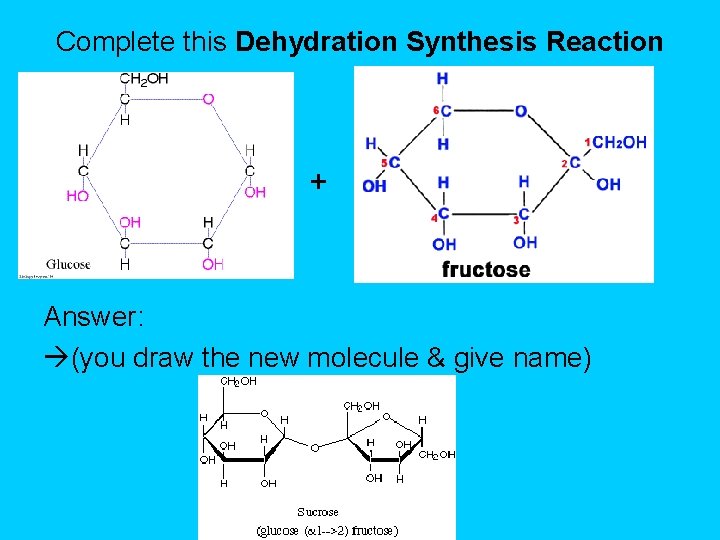
Complete this Dehydration Synthesis Reaction + Answer: (you draw the new molecule & give name)

Do Now • What is the element found in all organic compounds? – Carbon • What is the process called that links two monomers together to form a polymer? – Dehydration synthesis or condensation reaction • What is removed during the above process? – Water

Organic Compounds q There are 4 main classes of organic compounds which are essential to the life processes of all living things. Ø Carbohydrates Ø Proteins Ø Lipids Ø Nucleic Acids

I. Carbohydrates § Elements: C, H, O in 1: 2: 1 ratio (double hydrogen) § Main fuel provider and energy source of living things, used for structures in cells § Exists in 2 forms: (ring formation common) § Monosaccharides –monomer of carbs like sugars § Polysaccharides- polymer of carbs
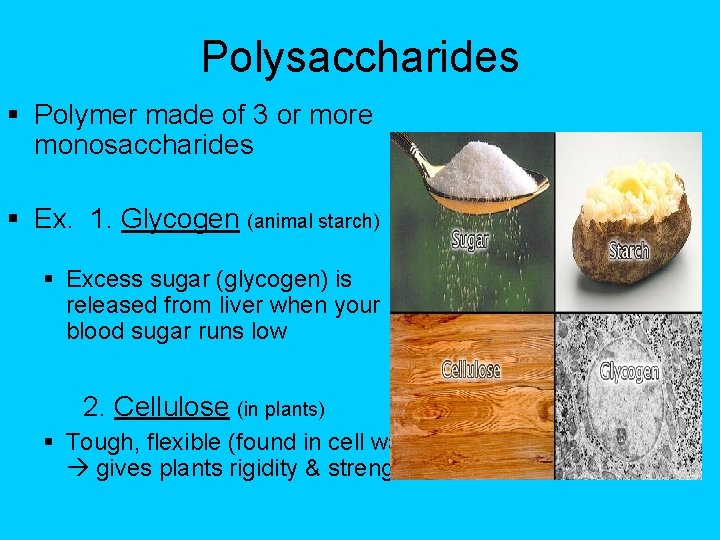
Polysaccharides § Polymer made of 3 or more monosaccharides § Ex. 1. Glycogen (animal starch) § Excess sugar (glycogen) is released from liver when your blood sugar runs low 2. Cellulose (in plants) § Tough, flexible (found in cell wall) gives plants rigidity & strength.
II. Proteins § Elements: C, H, O, N § For building bones and muscles, as well as cell membranes and enzymes § Monomer = Amino Acids (20 kinds) § Foods- Fish, poultry, soy, beans
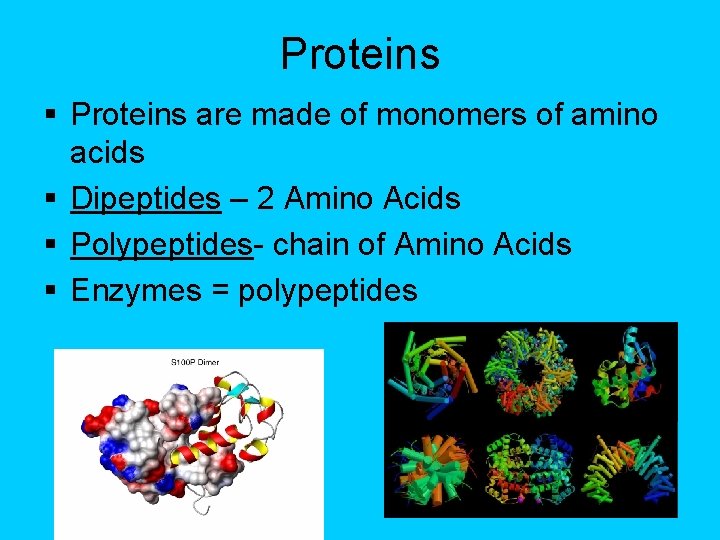
Proteins § Proteins are made of monomers of amino acids § Dipeptides – 2 Amino Acids § Polypeptides- chain of Amino Acids § Enzymes = polypeptides

III. Lipids § Elements: C, H (in high ratio) & O § NOT water soluble (do NOT dissolve in water) § Monomer = 1 glycerol + 3 fatty acids § Used to store energy. Important part in biological membranes and waterproof covering § Ex. Fats, oils, waxes – Fatty acids – Complex Lipids
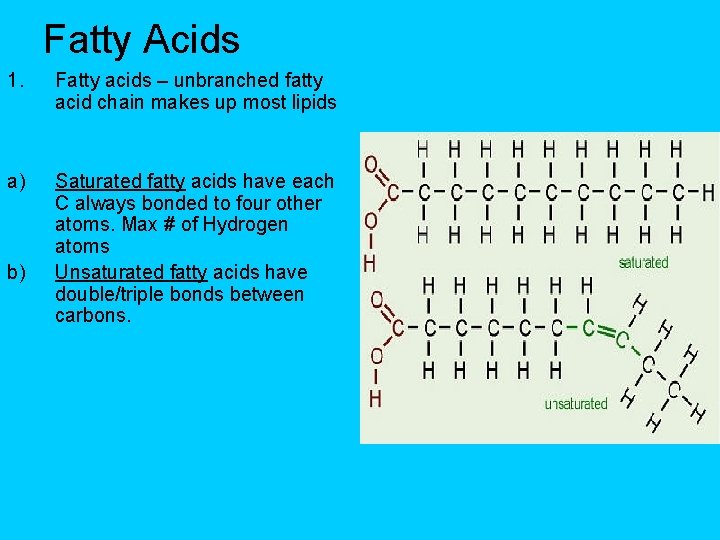
Fatty Acids 1. Fatty acids – unbranched fatty acid chain makes up most lipids a) Saturated fatty acids have each C always bonded to four other atoms. Max # of Hydrogen atoms Unsaturated fatty acids have double/triple bonds between carbons. b)
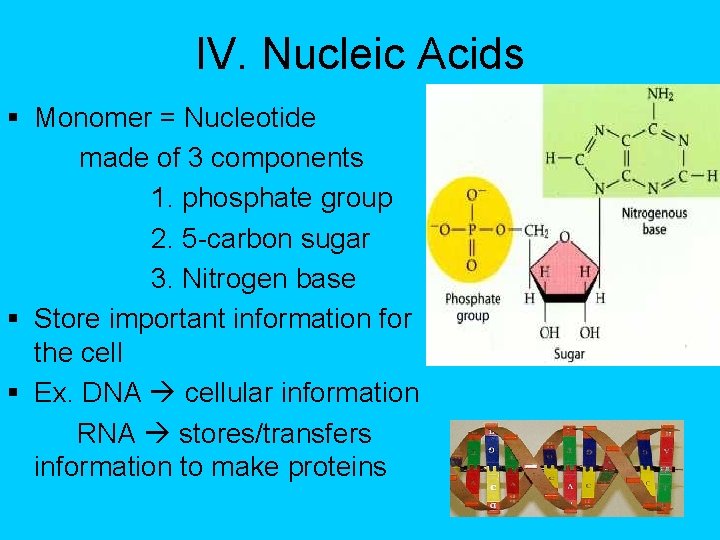
IV. Nucleic Acids § Monomer = Nucleotide made of 3 components 1. phosphate group 2. 5 -carbon sugar 3. Nitrogen base § Store important information for the cell § Ex. DNA cellular information RNA stores/transfers information to make proteins
- Slides: 17