Carbon Compounds 2 3 Carbon is versatile and

Carbon Compounds 2 -3

Carbon is versatile and is the “backbone” of life • Organic chemistry- the study of all compounds that contain bonds between carbon atoms • Carbon has four outer shell electrons, – makes four bonds Carbon can bond with many elements
Macromolecules • Carbon can form chains thousands of atoms long. • Chains of carbon atoms can also branch, and double and triple bond. • Macromolecules- GIANT MOLECULES.

Important Groups of Macromolecules 1. Carbohydrate Sugars Starches 2. Lipids 3. Proteins 4. Nucleic acids
I. Polymers • What is a polymer? • Poly = many; mer = part. A polymer is a large molecule consisting of many smaller sub-units bonded together. • What is a monomer? • A monomer is a sub-unit of a polymer.
II. Classes of Organic Molecules: • What are the four classes of organic molecules? • Carbohydrates • Lipids • Proteins • Nucleic Acids

1. Carbohydrates • 1. Sugars • Carbo = carbon, hydrate = water; carbohydrates have the molecular formula (CH 2 O)n • Functions: • Store energy in chemical bonds • Glucose is the most common monosaccharide • Glucose is produced by photosynthesis
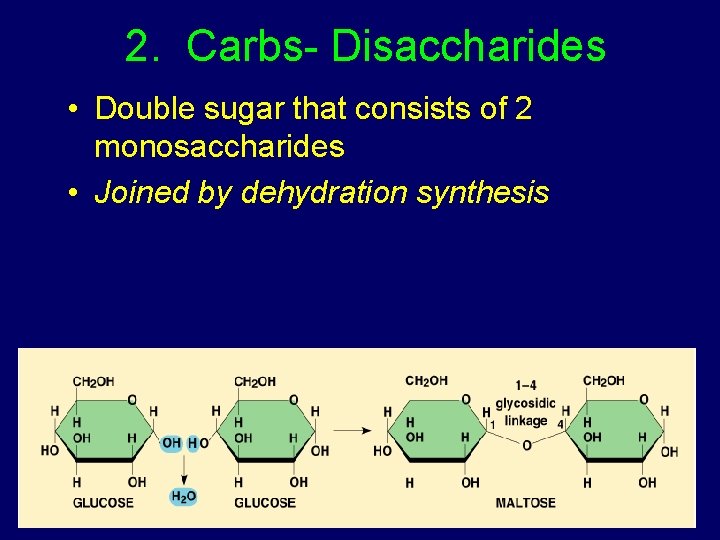
2. Carbs- Disaccharides • Double sugar that consists of 2 monosaccharides • Joined by dehydration synthesis
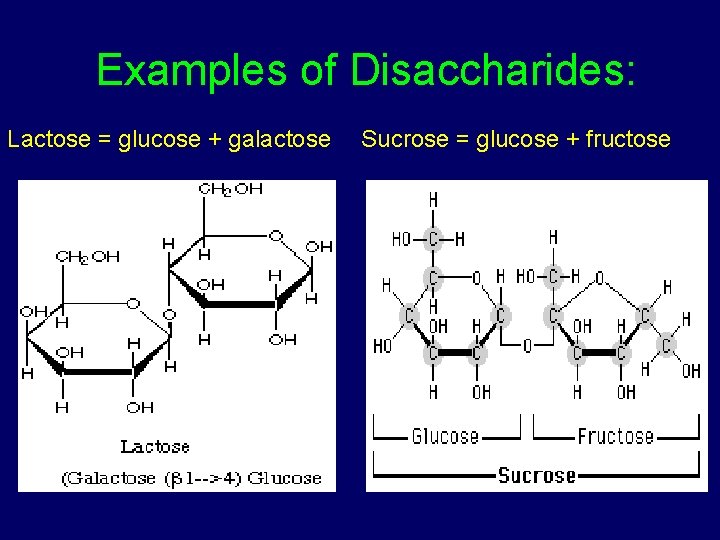
Examples of Disaccharides: Lactose = glucose + galactose Sucrose = glucose + fructose
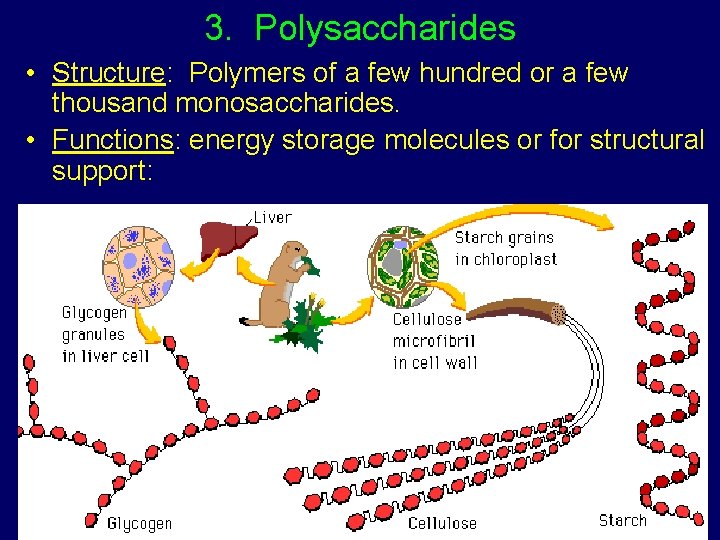
3. Polysaccharides • Structure: Polymers of a few hundred or a few thousand monosaccharides. • Functions: energy storage molecules or for structural support:

2. Lipids • Structure: Greasy or oily nonpolar compounds. Many composed of glycerol and fatty acids. • Functions: • Energy storage • membrane structure • Protecting against desiccation (drying out). • Insulating against cold. • Regulating cell activities by hormone actions.

Saturated and Unsaturated Fats • Unsaturated fats : – liquid at room temp – one or more double bonds between carbons in the fatty acids allows for “kinks” in the tails – most plant fats • Saturated fats: – have only single C-C bonds in fatty acid tails – solid at room temp – most animal fats
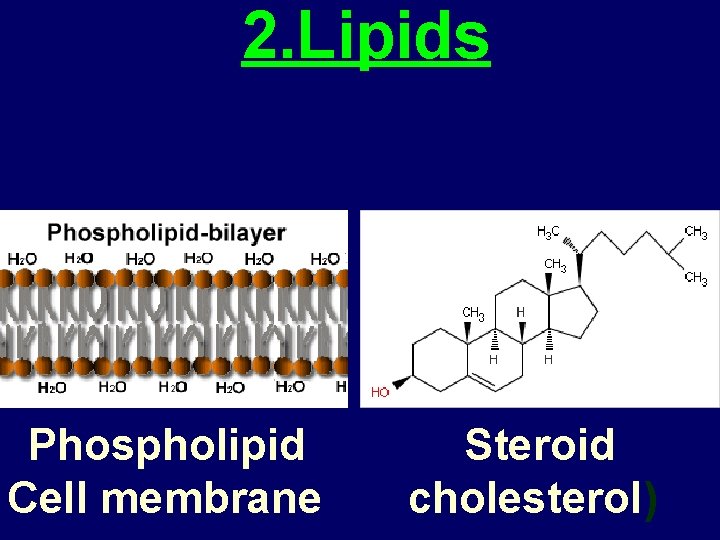
2. Lipids Phospholipid Cell membrane Steroid cholesterol)

3. Proteins • Structure: • Polypeptide chains • Consist of peptide bonds between 20 possible amino acid monomers • Have a 3 dimensional globular shape
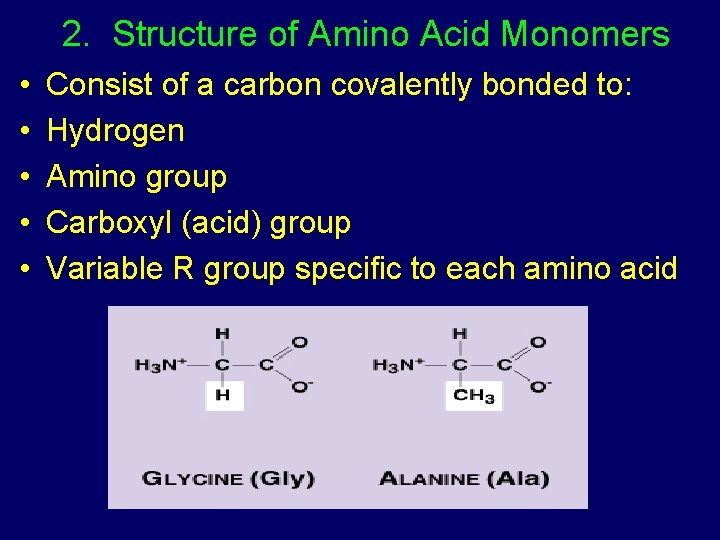
2. Structure of Amino Acid Monomers • • • Consist of a carbon covalently bonded to: Hydrogen Amino group Carboxyl (acid) group Variable R group specific to each amino acid
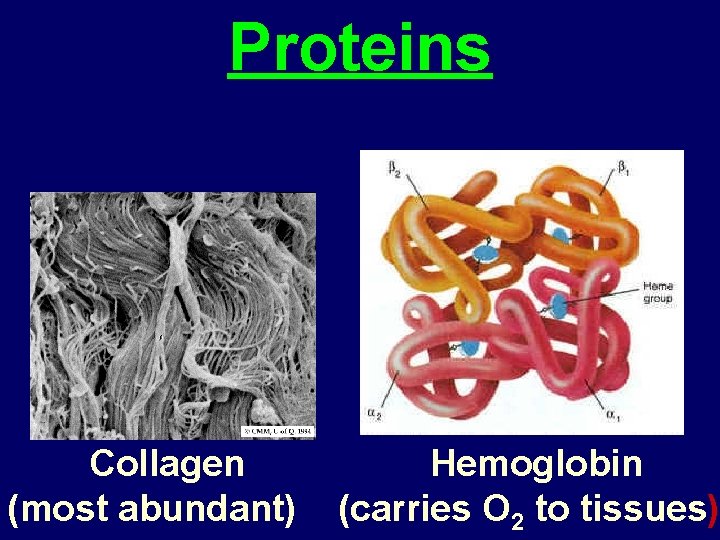
Proteins Collagen (most abundant) Hemoglobin (carries O 2 to tissues)
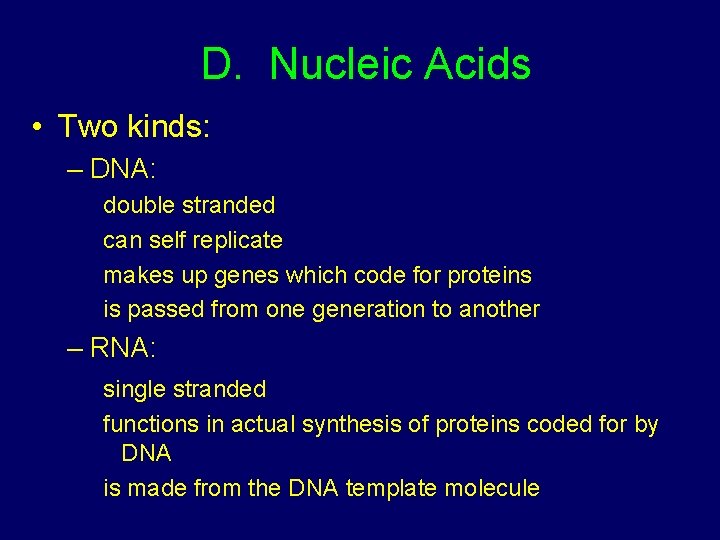
D. Nucleic Acids • Two kinds: – DNA: double stranded can self replicate makes up genes which code for proteins is passed from one generation to another – RNA: single stranded functions in actual synthesis of proteins coded for by DNA is made from the DNA template molecule
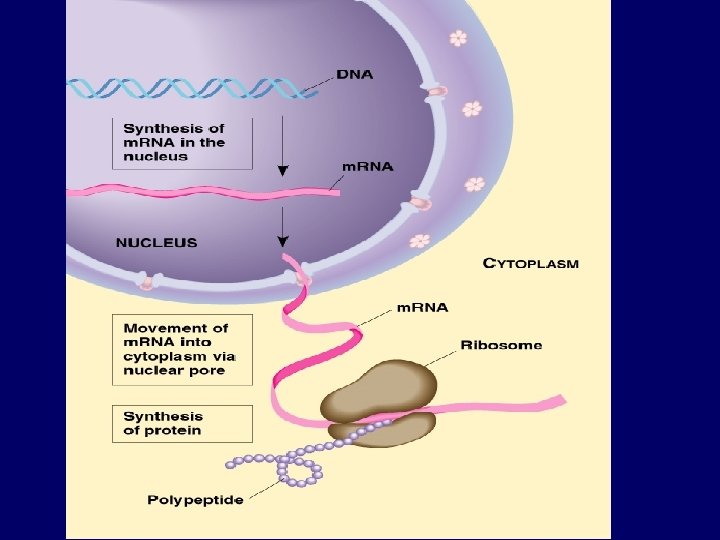
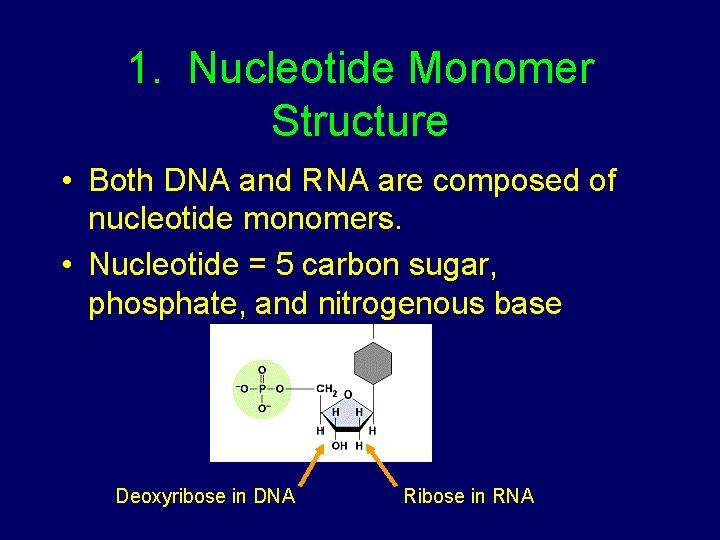
1. Nucleotide Monomer Structure • Both DNA and RNA are composed of nucleotide monomers. • Nucleotide = 5 carbon sugar, phosphate, and nitrogenous base Deoxyribose in DNA Ribose in RNA
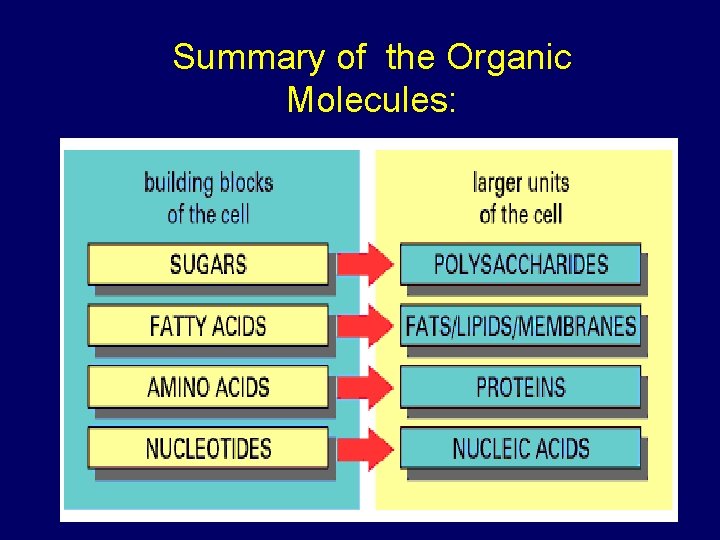
Summary of the Organic Molecules:
1. Functions of Proteins 1. Enzymes which accelerate specific chemical reactions up to 10 billion times faster. 2. Structural materials, including keratin (the protein found in hair and nails) collagen (the protein found in connective tissue), and myosin (found in muscles).

3. Antibodies that bind specifically to foreign substances to identify them to the body's immune system. 4. Specific carriers, including membrane transport proteins that move substances across cell membranes, and blood proteins, such as hemoglobin, that carry oxygen, iron, and other substances through the body.

5. Contraction, such as actin and myosin fibers that interact in muscle tissue. 6. Signaling, including hormones such as insulin that regulate sugar levels in blood.
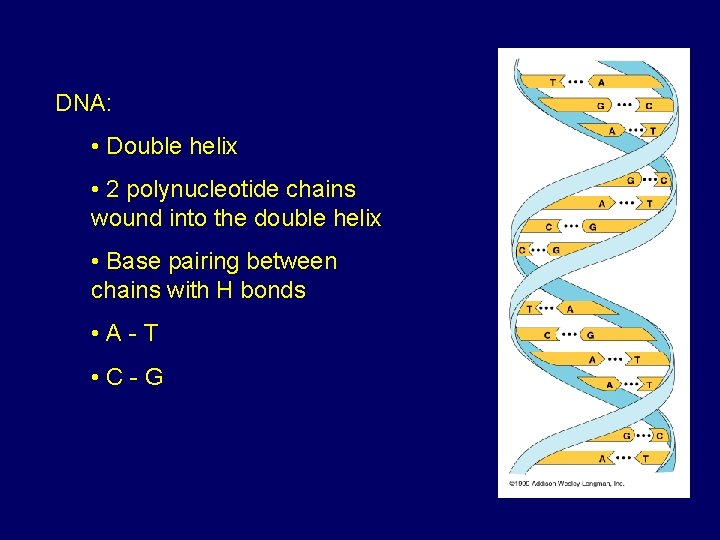
DNA: • Double helix • 2 polynucleotide chains wound into the double helix • Base pairing between chains with H bonds • A-T • C-G

ENZYMES

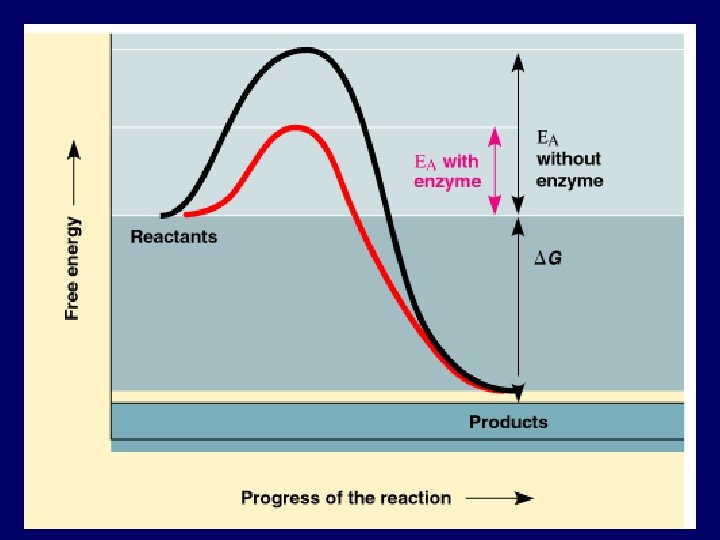
How Do Reactions Occur? • Spontaneous reactions may occur very slowly. • All reactions require free energy of activation (EA) • Uphill portion represents the EA required to start the reaction. • Downhill portion represents the loss of free energy by the molecules in the reaction. • DG is the difference in free energy of products and reactants.
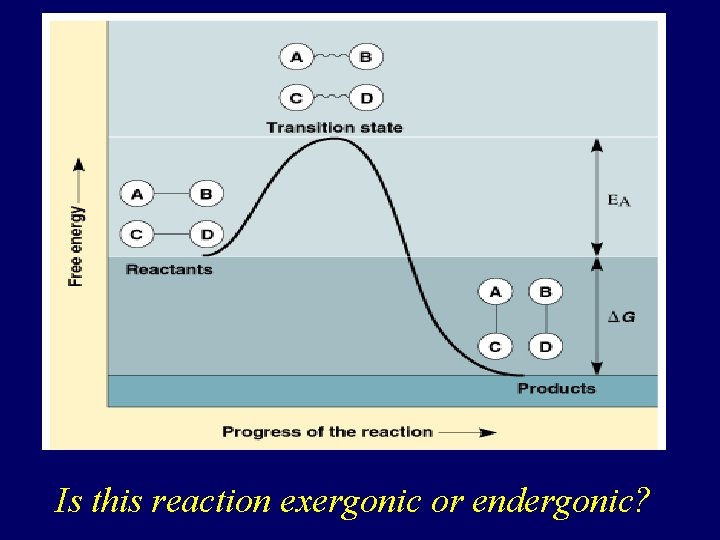
Is this reaction exergonic or endergonic?

How can the EA barrier be overcome? • Temperatures that are too high denature organic molecules, so what else is there? • ENZYMES • Enzymes lower the EA barrier so that reactions can occur at lower temperatures.
What are Enzymes? • Catalysts change the rate of the reaction without being altered themselves. • Enzymes are biological catalysts. • Enzymes are proteins, whose three dimensional shape allows for their ability to react specifically.
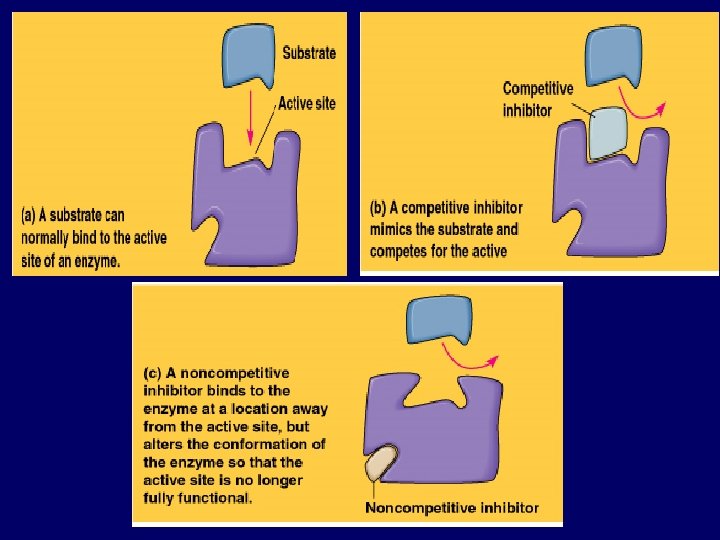
Enzyme / Substrate Relationship: • What is the substrate? • It is the reactant upon which an enzyme reacts. • Enzymes are substrate specific. • Only the active site of the enzyme actually binds the substrate.
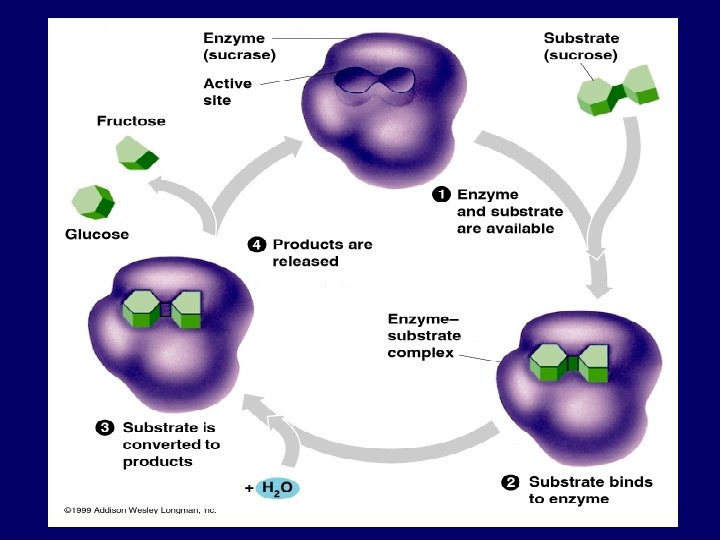

The Active Site • The active site is where the enzymesubstrate interaction occurs. • Most enzyme-substrate interactions are the result of weak bonds. • The active site may cause the enzyme to hold onto the substrate in a very specific way. • The active site may provide a microenvironment (e. g. low p. H) which enhances a reaction.

Enzyme Activity • Temperature • p. H • Enzyme Concentration • Substrate Concentration
Cofactors • Non-protein molecules that help enzymes function. • Bind to active site to enhance enzymatic reactions. • Cofactors may be inorganic metals such as zinc, iron, or copper. • Coenzymes are organic cofactors (e. g. vitamins)

Enzyme Inhibition • Competitive inhibitors - mimic the substrate and compete for the active site. • Non-competitive inhibitors bind to the enzyme away from the active site, and indirectly cause a change in the active site.
- Slides: 37