Carbon Chemistry Is Carbon Important Found in all

Carbon Chemistry

Is Carbon Important? • Found in all living matter • Used in … Body Tissues Food Coal Wood

Diamond • A colorless, crystalline, solid form of carbon • Hardest material known • Used for cutting, drilling, and grinding • High melting point (3500°C)
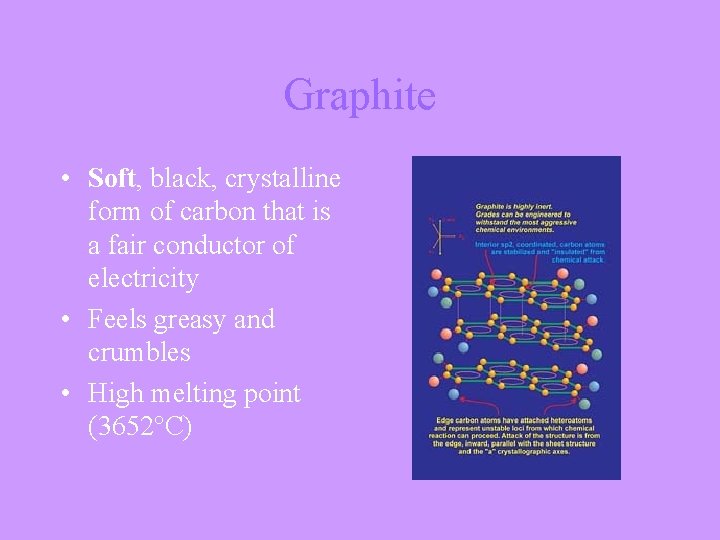
Graphite • Soft, black, crystalline form of carbon that is a fair conductor of electricity • Feels greasy and crumbles • High melting point (3652°C)
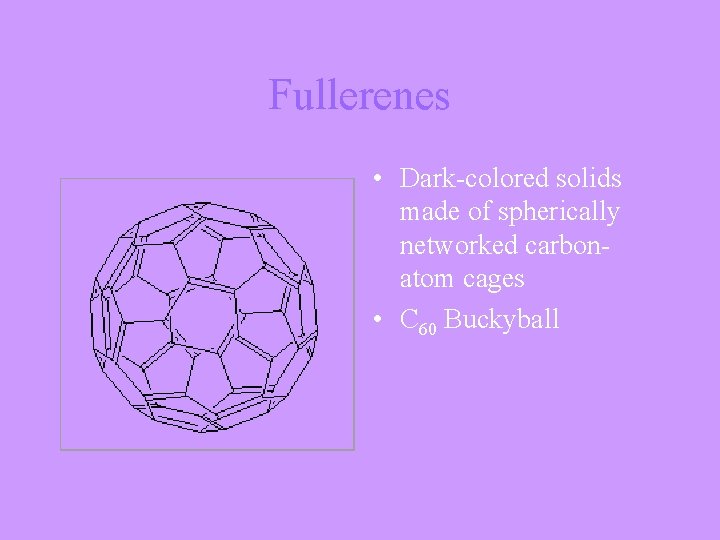
Fullerenes • Dark-colored solids made of spherically networked carbonatom cages • C 60 Buckyball

Organic Compounds • Covalently bonded compounds containing carbon, excluding carbonates and oxides • All organic compounds contain carbon • Not Na 2 CO 3, CO, and CO 2 • Aspirin, Citric Acid, Plastic Bags, Amino Acids
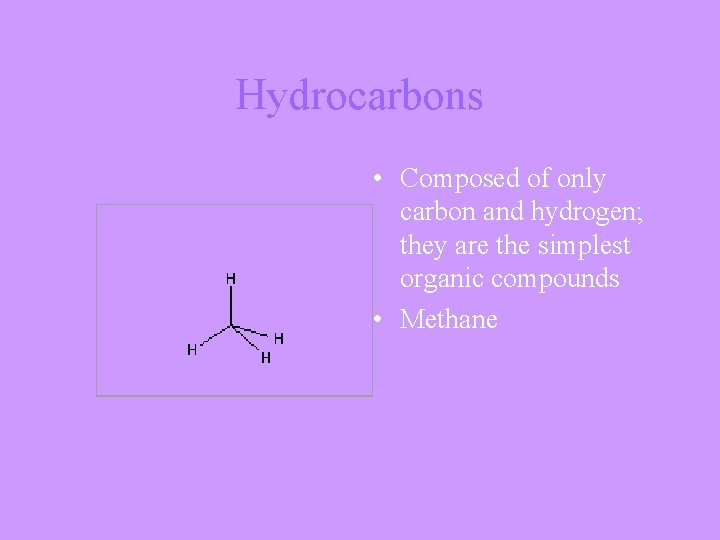
Hydrocarbons • Composed of only carbon and hydrogen; they are the simplest organic compounds • Methane
Structures • Isomers-Have the same molecular formula but different structures • Structural isomers-isomers in which the atoms are bonded together in different orders • Geometric isomers-isomers in which the order or atom bonding is the same but the arrangement of atoms in space is different • p 631 -632
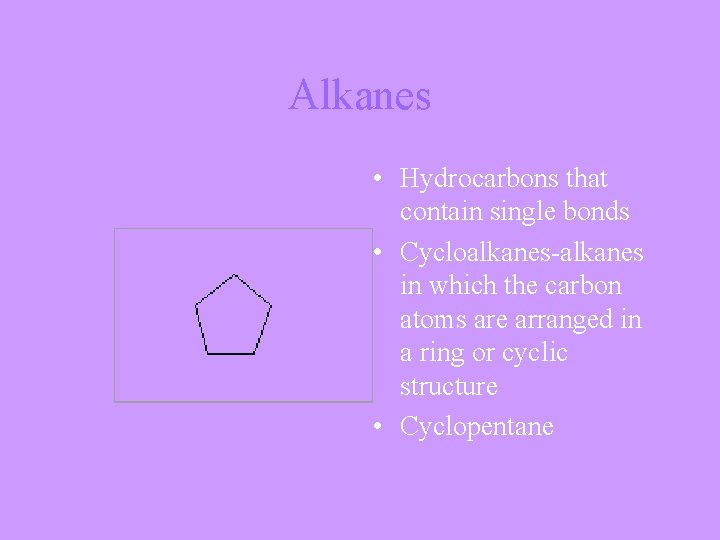
Alkanes • Hydrocarbons that contain single bonds • Cycloalkanes-alkanes in which the carbon atoms are arranged in a ring or cyclic structure • Cyclopentane
Naming Alkanes • • Meth- 1 Eth 2 Prop- 3 But- 4 Pent- 5 Hex- 6 Oct- 8 Dec- 10 • Count the number of carbons and add -ane

Uses of Alkanes • Natural gas • Paraffin wax • Fractional distillation-separates petroleum based on boiling point

Alkenes • Hydrocarbons that contain double covalent bonds • Ethene-used in making plastics, plant hormone • Prefix(carbon bonds) + ene • Found in the natural wax of apples

Alkynes • Hydrocarbons with triple covalent bonds • Ethyne-used in welding torches • Prefix(# of C) + yne
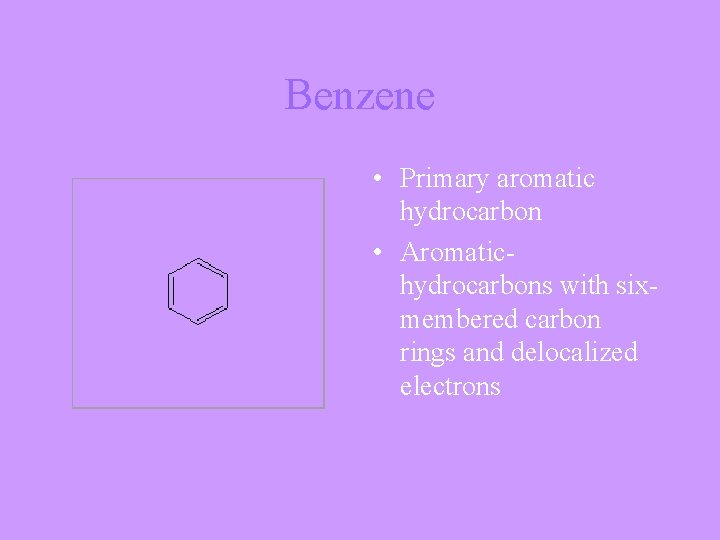
Benzene • Primary aromatic hydrocarbon • Aromatichydrocarbons with sixmembered carbon rings and delocalized electrons

Alcohols • Organic compounds that contain one or more hydroxyl (OH) groups • Naming: Carbon # and add –ol p 664
Polymers • Large molecules made up of many small units joined to each other through organic reactions • p 687 Polystyrene
- Slides: 16