CARBON CHEMISTRY Hydrocarbons organic compound that contains C
CARBON CHEMISTRY
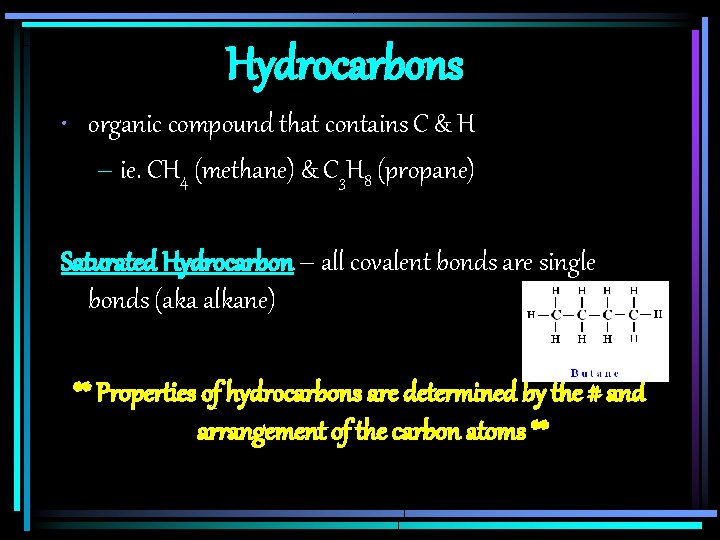
Hydrocarbons • organic compound that contains C & H – ie. CH 4 (methane) & C 3 H 8 (propane) Saturated Hydrocarbon – all covalent bonds are single bonds (aka alkane) ** Properties of hydrocarbons are determined by the # and arrangement of the carbon atoms **
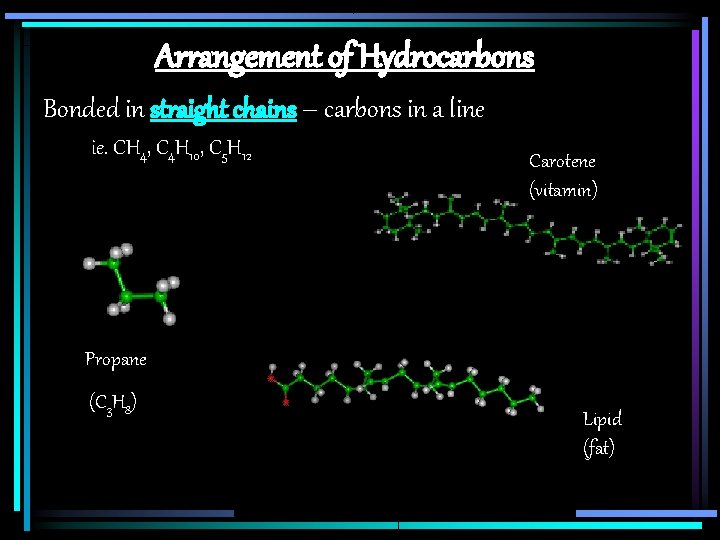
Arrangement of Hydrocarbons Bonded in straight chains – carbons in a line ie. CH 4, C 4 H 10, C 5 H 12 Carotene (vitamin) Propane (C 3 H 8) Lipid (fat)
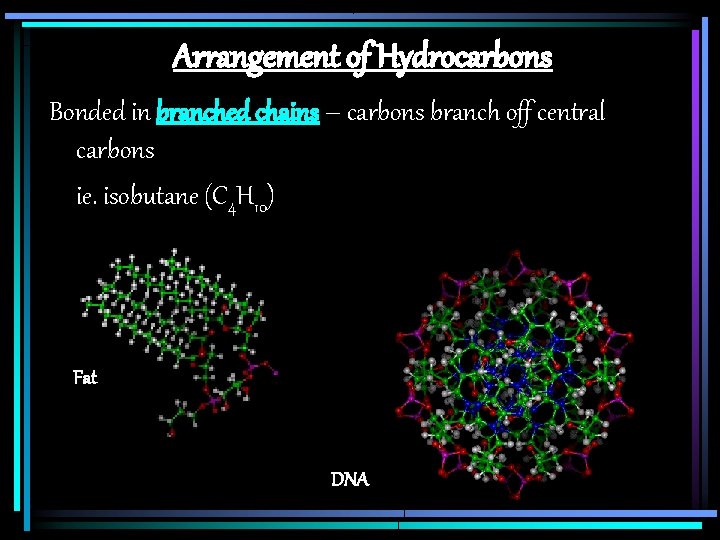
Arrangement of Hydrocarbons Bonded in branched chains – carbons branch off central carbons ie. isobutane (C 4 H 10) Fat DNA
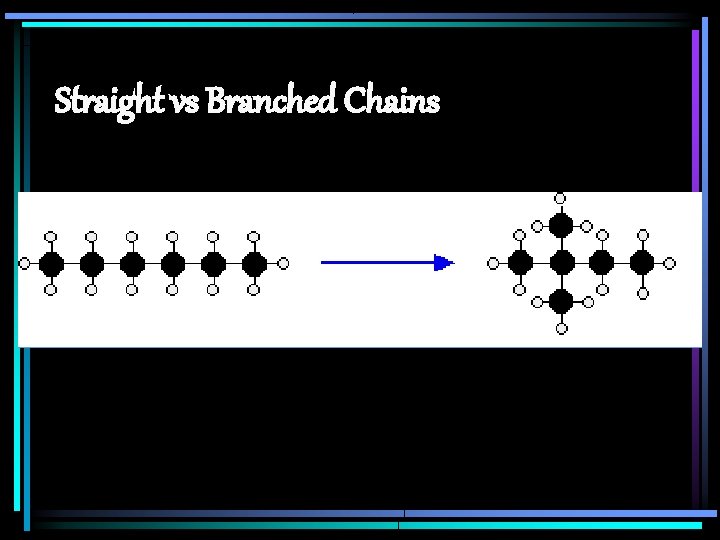
Straight vs Branched Chains

STOP for Vocabulary Review! Isomer – compounds with the same molecular formula but different structural formulas ie. C 4 H 10 (butane & iso-butane)
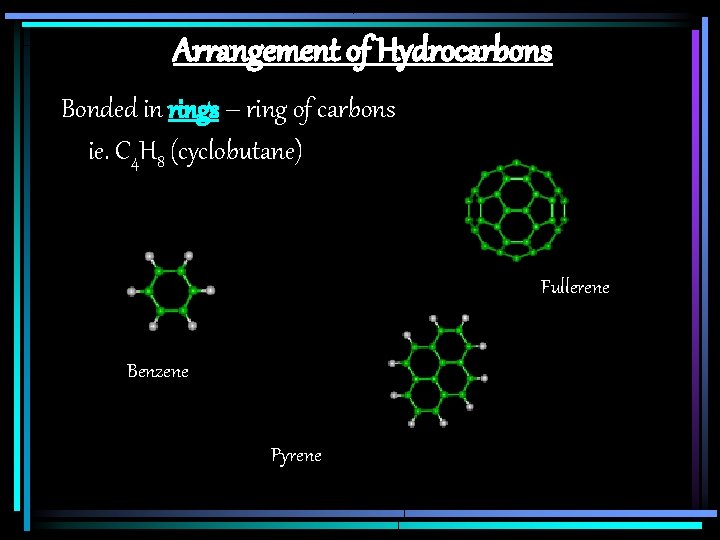
Arrangement of Hydrocarbons Bonded in rings – ring of carbons ie. C 4 H 8 (cyclobutane) Fullerene Benzene Pyrene
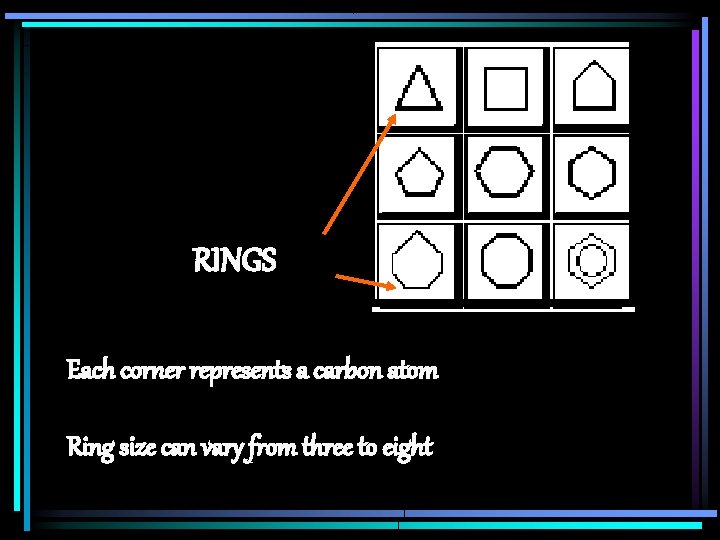
RINGS Each corner represents a carbon atom Ring size can vary from three to eight
Unsaturated Hydrocarbons • a hydrocarbon that contains one or more double or triple bonds Types of unsaturated hydrocarbons – Alkene – double bonded carbons • ie. C 2 H 4 (ethylene) – Alkyne – straight or branched carbons with triple bonds • ie. C 2 H 2 (ethyne) – Aromatic – rings with a distinct odor
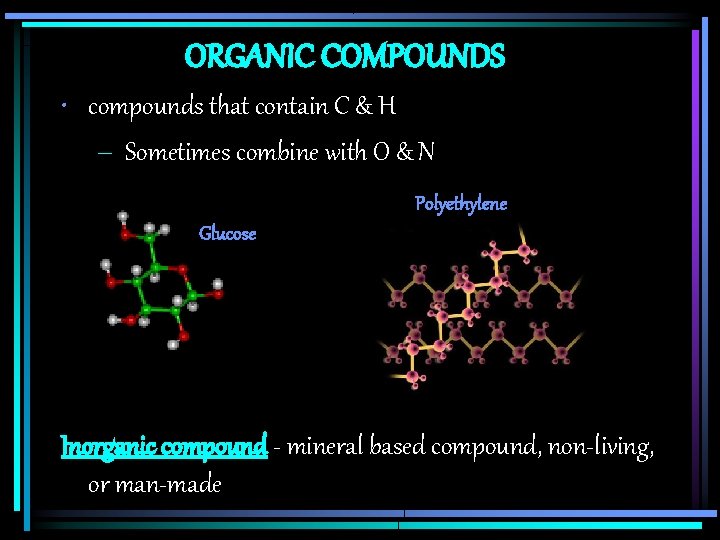
ORGANIC COMPOUNDS • compounds that contain C & H – Sometimes combine with O & N Polyethylene Glucose Inorganic compound - mineral based compound, non-living, or man-made
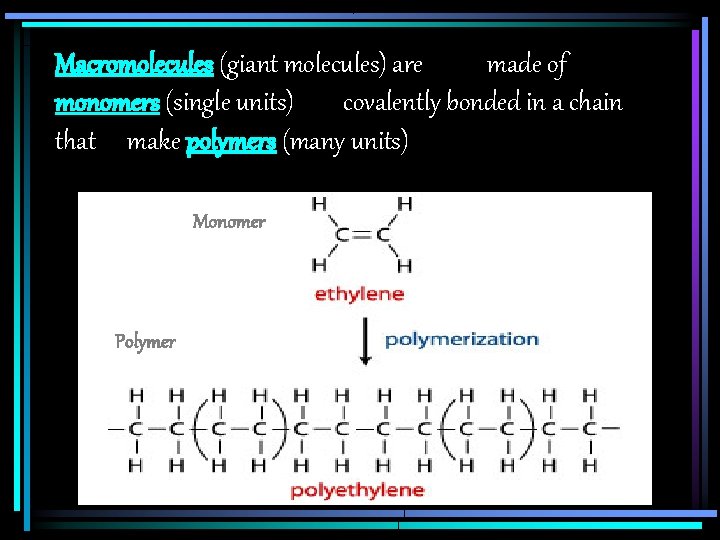
Macromolecules (giant molecules) are made of monomers (single units) covalently bonded in a chain that make polymers (many units) Monomer Polymer
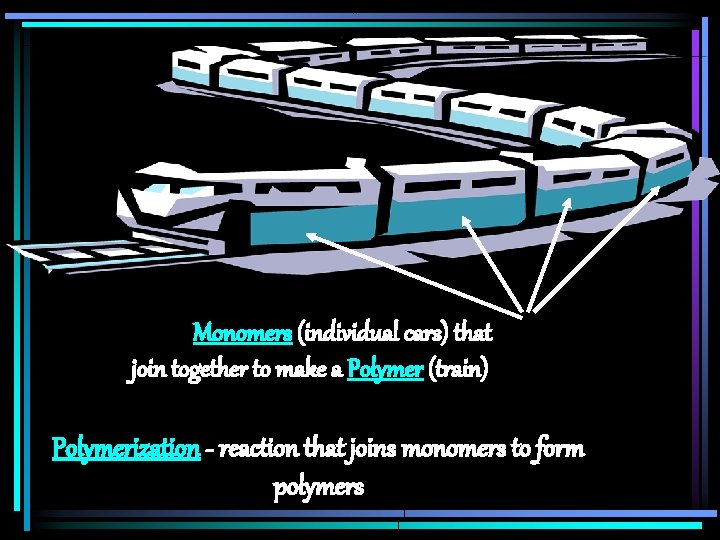
Monomers (individual cars) that join together to make a Polymer (train) Polymerization - reaction that joins monomers to form polymers
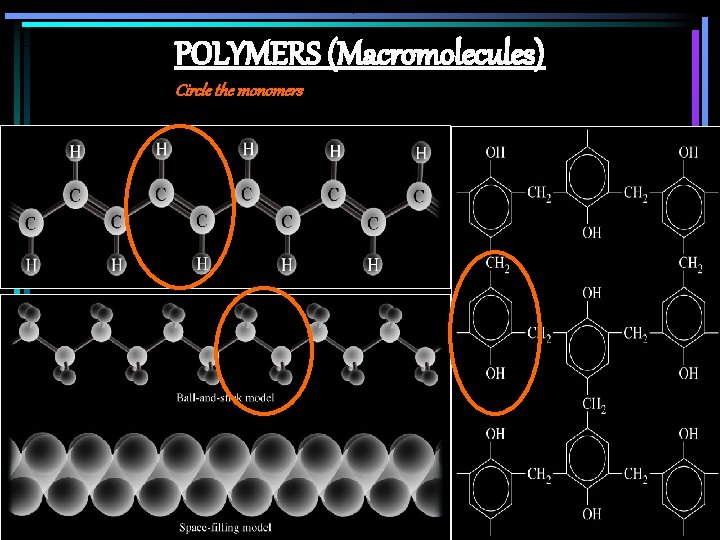
POLYMERS (Macromolecules) Circle the monomers

Types of Polymers Synthetic – man-made Rubber – tires & adhesives Nylon – parachutes, windbreakers, fishing line, carpets & ropes Polyethylene – chains of ethylene • milk jugs, plastic wrap & most other plastic items (toys, etc. )
Types of Polymers Natural - (contain C, H & O) Starch – glucose, bread, pasta Carbo (C) / Hydrate (H 2 O) Cellulose – found in plants 3000+ glucose in a chain Nucleic Acid – DNA & RNA stores information in cells Protein – muscle, hair, nails, foods 100+ amino acids in a chain
Carbon-Based Polymers Your task: • Using your notes, labs or the internet identify TWO synthetic, large, carbon-based molecules • The molecules must be MORE complex than a simple hydrocarbon such as C 8 H 18 • Your research must include a structural formula for each molecule • Be sure to write down your sources

Fossil Fuels • mixtures of hydrocarbons that formed from the remains of plants or animals • Burning of fossil fuels contributes to Acid Rain – precipitation that has a lower than normal p. H (<5. 6) • Contains sulfuric & nitric acid
Types of Fossil Fuels Coal – solid formed about 300 mya • • Made from swamp plants Mostly aromatic hydrocarbons that produce more soot than other fossil fuels Natural Gas – gas formed from the remains of marine organisms • • Mostly CH 4 Used for households & to generate electricity Petroleum – crude oil formed from the remains of marine organisms • • Long branched alkanes & alkenes Must be separated to be useful (ie. gasoline & heating oil)
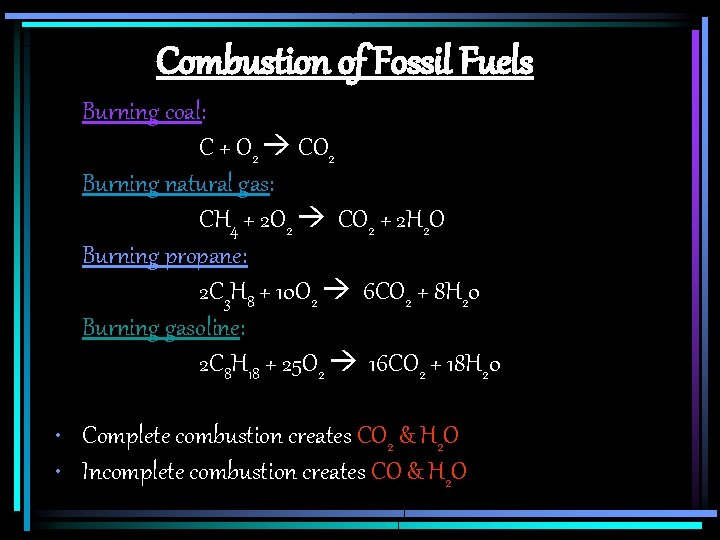
Combustion of Fossil Fuels Burning coal: C + O 2 CO 2 Burning natural gas: CH 4 + 2 O 2 CO 2 + 2 H 2 O Burning propane: 2 C 3 H 8 + 10 O 2 6 CO 2 + 8 H 20 Burning gasoline: 2 C 8 H 18 + 25 O 2 16 CO 2 + 18 H 20 • Complete combustion creates CO 2 & H 2 O • Incomplete combustion creates CO & H 2 O

Video NPR video on Carbon http: //www. npr. org/news/specials/climate/video/ Works Cited • • geo. arc. nasa. gov www. hibbing. tec. mn. us www. richmond. gov. uk lakes. chebucto. org
- Slides: 20