Carbon atoms Common name IUPAC name Chemical formula

Carbon atoms Common name IUPAC name Chemical formula Common location or use 1 Formic acid Methanoic acid HCOOH Insect stings 2 Acetic acid Ethanoic acid CH 3 COOH Vinegar 3 Propionic acid Propanoic acid CH 3 CH 2 COOH 4 Butyric acid Butanoic acid CH 3(CH 2)2 COOH Rancid butter 5 Valeric acid Pentanoic acid CH 3(CH 2)3 COOH Valerian 6 Caproic acid Hexanoic acid CH 3(CH 2)4 COOH 7 Enanthic acid Heptanoic acid CH 3(CH 2)5 COOH 8 Caprylic acid Octanoic acid CH 3(CH 2)6 COOH Coconuts and breast milk 9 Pelargonic acid Nonanoic acid CH 3(CH 2)7 COOH Pelargonium 10 Capric acid Decanoic acid CH 3(CH 2)8 COOH 12 Lauric acid Dodecanoic acid CH 3(CH 2)10 COOH 16 Palmitic acid Hexadecanoic acid CH 3(CH 2)14 COOH Palm oil 18 Stearic acid Octadecanoic acid Some waxes, soaps CH 3(CH 2)16 COOH Coconut oil

Acrylic acid (2 -propenoic acid) – CH 2=CHCOOH, used in polymer synthesis Fatty acids – medium to long-chain saturated and unsaturated monocarboxylic acids, with even number of carbons Docosahexaenoic acid – nutritional supplement Eicosapentaenoic acid – nutritional supplement Amino acids – the building blocks of proteins Keto acids – acids of biochemical significance that contain a ketone group Pyruvic acid Acetoacetic acid Aromatic carboxylic acids Benzoic acid – C 6 H 5 COOH; sodium benzoate, the sodium salt of benzoic acid is used as a food preservative Salicylic acid – found in many skin care products Dicarboxylic acids – containing two carboxyl groups Aldaric acid – a family of sugar acids Oxalic acid – found in many foods Malonic acid Malic acid – found in apples Fumaric acid Succinic acid – a component of the citric acid cycle Glutaric acid Adipic acid – the monomer used to produce nylon Tricarboxylic acids – containing three carboxyl groups Citric acid – found in citrus fruits Isocitric acid Aconitic acid Propane-1, 2, 3 -tricarboxylic acid (tricarballylic acid, carballylic acid) Alpha hydroxy acids – containing a hydroxy group Lactic acid (2 -hydroxypropanoic acid) – found in sour milk
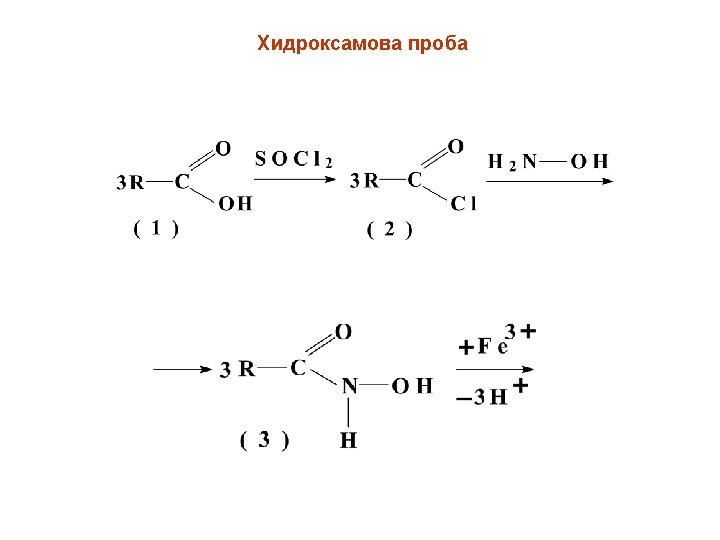
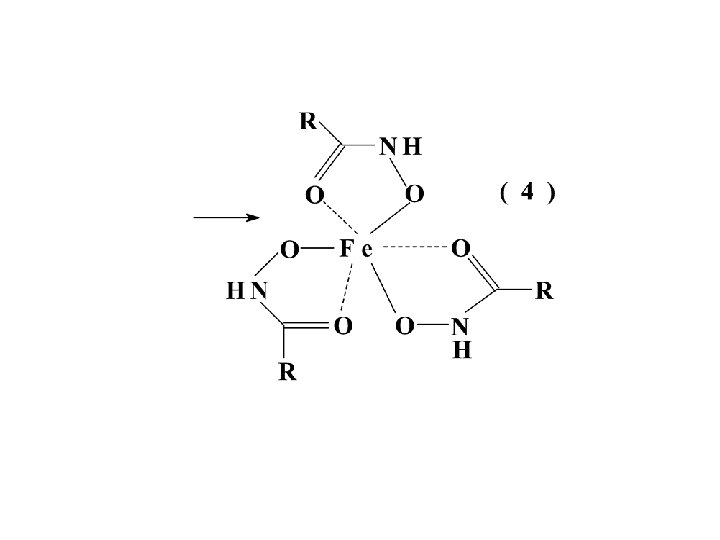
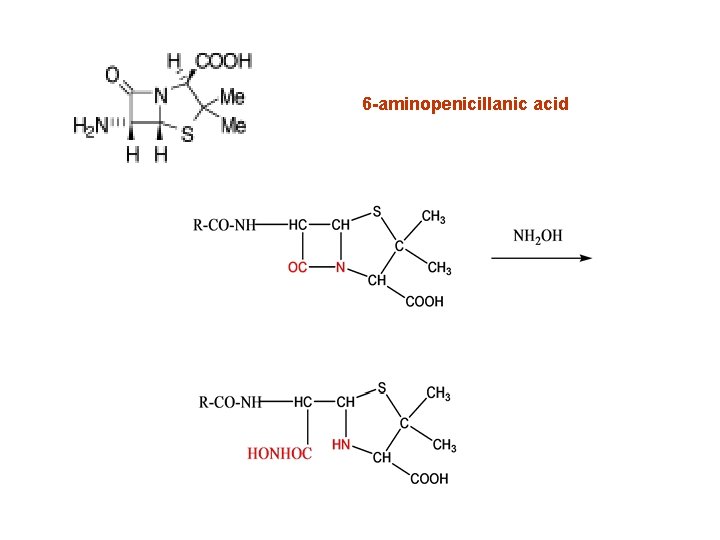
6 -aminopenicillanic acid
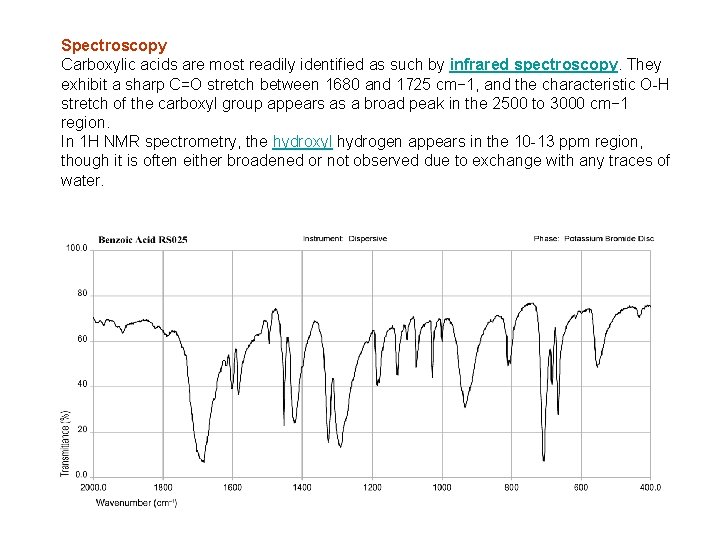
Spectroscopy Carboxylic acids are most readily identified as such by infrared spectroscopy. They exhibit a sharp C=O stretch between 1680 and 1725 cm− 1, and the characteristic O-H stretch of the carboxyl group appears as a broad peak in the 2500 to 3000 cm− 1 region. In 1 H NMR spectrometry, the hydroxyl hydrogen appears in the 10 -13 ppm region, though it is often either broadened or not observed due to exchange with any traces of water.
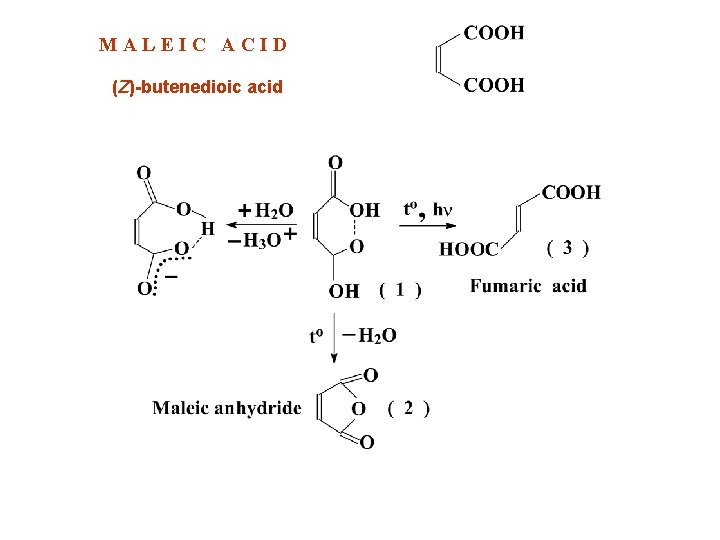
MALEIC ACID (Z)-butenedioic acid
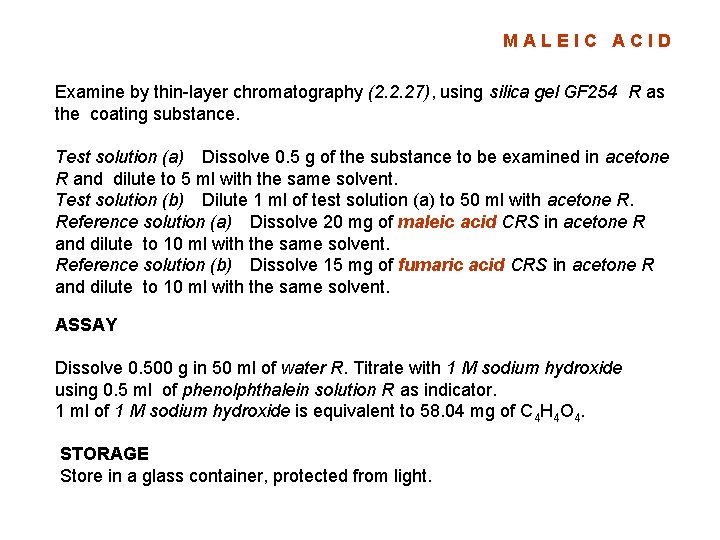
MALEIC ACID Examine by thin-layer chromatography (2. 2. 27), using silica gel GF 254 R as the coating substance. Test solution (a) Dissolve 0. 5 g of the substance to be examined in acetone R and dilute to 5 ml with the same solvent. Test solution (b) Dilute 1 ml of test solution (a) to 50 ml with acetone R. Reference solution (a) Dissolve 20 mg of maleic acid CRS in acetone R and dilute to 10 ml with the same solvent. Reference solution (b) Dissolve 15 mg of fumaric acid CRS in acetone R and dilute to 10 ml with the same solvent. ASSAY Dissolve 0. 500 g in 50 ml of water R. Titrate with 1 M sodium hydroxide using 0. 5 ml of phenolphthalein solution R as indicator. 1 ml of 1 M sodium hydroxide is equivalent to 58. 04 mg of C 4 H 4 O 4. STORAGE Store in a glass container, protected from light.
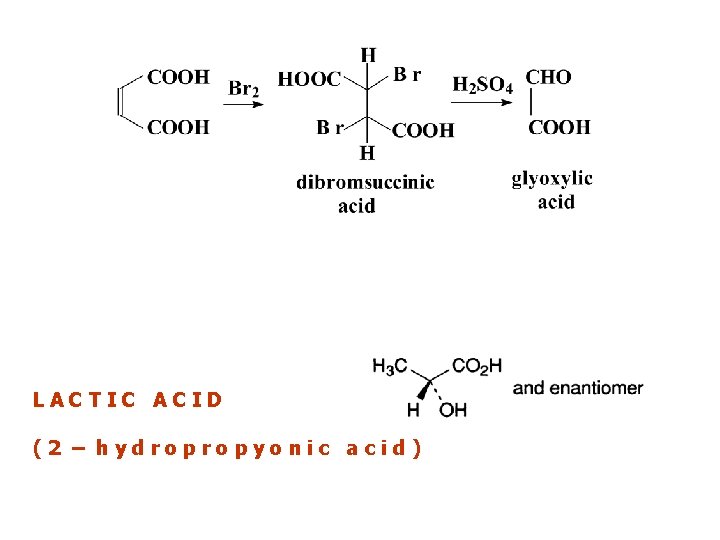
LACTIC ACID (2 – hydropropyonic acid)

LACTIC ACID Lactates Dissolve a quantity of the substance to be examined equivalent to about 5 mg of lactic acid in 5 ml of water R or use 5 ml of the prescribed solution. Add 1 ml of bromine water R and 0. 5 ml of dilute sulphuric acid R. Heat on a water-bath until the colour is discharged, stirring occasionally with a glass rod. Add 4 g of ammonium sulphate R and mix. Add dropwise and without mixing 0. 2 ml of a 100 g/l solution of sodium nitroprusside R in dilute sulphuric acid R. Still without mixing add 1 ml of concentrated ammonia R. Allow to stand for 30 min. A dark green ring appears at the junction of the two liquids.

LACTIC ACID

LACTIC ACID Sugars and other reducing substances To 1 ml of solution S add 1 ml of 1 M hydrochloric acid, heat to boiling, allow to cool and add 1. 5 ml of 1 M sodium hydroxide and 2 ml of cupritartaric solution R. Heat to boiling. No red or greenish precipitate is formed. Methanol (2. 4. 24) Maximum 50 ppm, if intended for use in the manufacture of parenteral dosage forms. Citric, oxalic and phosphoric acids To 5 ml of solution S add dilute ammonia R 1 until slightly alkaline (2. 2. 4). Add 1 ml of calcium chloride solution R. Heat on a water-bath for 5 min. Both before and after heating, any opalescence in the solution is not more intense than that in a mixture of 1 ml of water R and 5 ml of solution S.

2 -hydroxypropane-1, 2, 3 -tricarboxylic acid CITRIC ACID
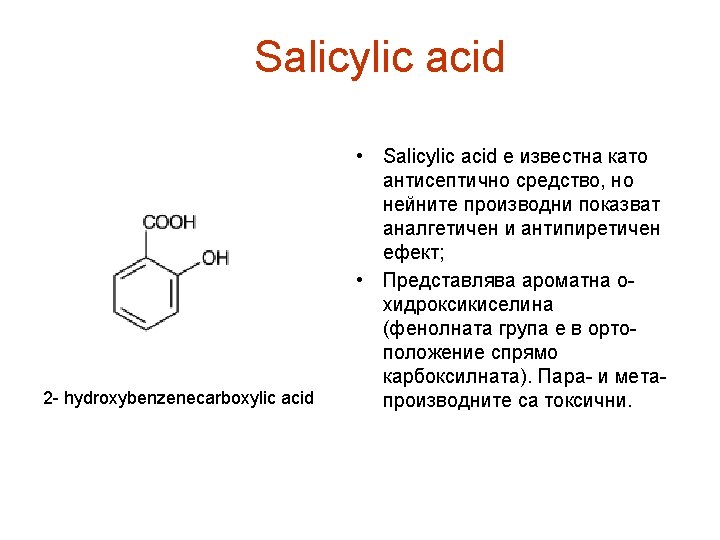

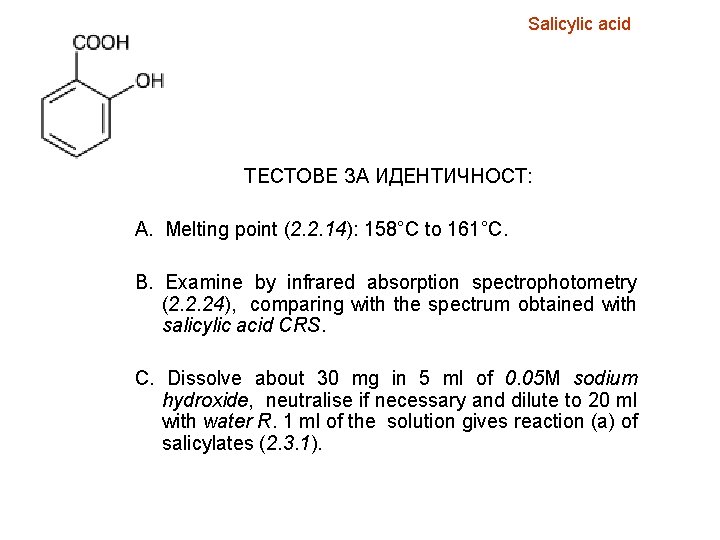
Salicylic acid ТЕСТОВЕ ЗА ИДЕНТИЧНОСТ: A. Melting point (2. 2. 14): 158°C to 161°C. B. Examine by infrared absorption spectrophotometry (2. 2. 24), comparing with the spectrum obtained with salicylic acid CRS. C. Dissolve about 30 mg in 5 ml of 0. 05 M sodium hydroxide, neutralise if necessary and dilute to 20 ml with water R. 1 ml of the solution gives reaction (a) of salicylates (2. 3. 1).
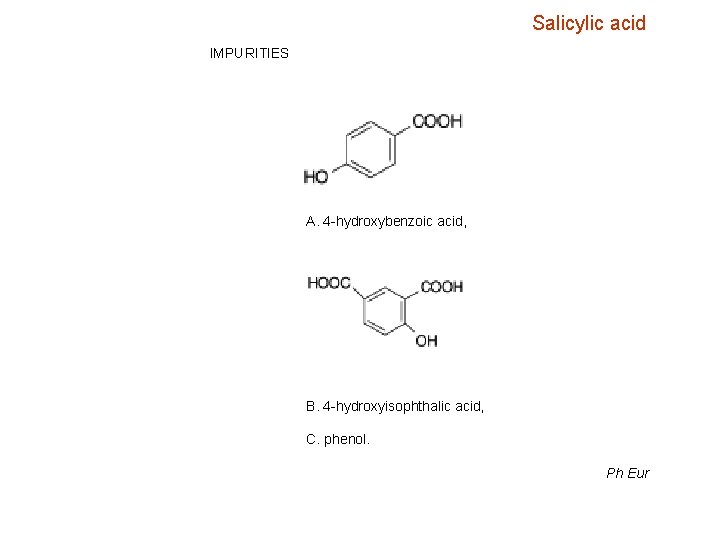
Salicylic acid IMPURITIES A. 4 -hydroxybenzoic acid, B. 4 -hydroxyisophthalic acid, C. phenol. Ph Eur

Salicylic acid Related substances Examine by liquid chromatography (2. 2. 29). Test solution. Dissolve 0. 50 g of the substance to be examined in the mobile phase and dilute to 100. 0 ml with the mobile phase. Reference solution (a). Dissolve 10 mg of phenol R in the mobile phase and dilute to 100. 0 ml with the mobile phase. Reference solution (b). Dissolve 25 mg of 4 -hydroxyisophthalic acid R in the mobile phase and dilute to 100. 0 ml with the mobile phase. Reference solution (c). Dissolve 50 mg of 4 -hydroxybenzoic acid R in the mobile phase and dilute to 100. 0 ml with the mobile phase. Reference solution (d). Dilute 1. 0 ml of reference solution (a) to 10. 0 ml with the mobile phase. Reference solution (e). Dilute a mixture of 1. 0 ml of each of reference solutions (a), (b) and (c) to 10. 0 ml with the mobile phase. Reference solution (f). Dilute a mixture of 0. 1 ml of each of reference solutions (a), (b) and (c) to 10. 0 ml with the mobile phase. The chromatographic procedure may be carried out using: — a stainless steel column 0. 15 m long and 4. 6 mm in internal diameter packed with non-deactivated octadecylsilyl silica gel for chromatography R (5 µm), — as mobile phase at a flow rate of 0. 5 ml per minute a mixture of 1 volume of glacial acetic acid R, 40 volumes of methanol R and 60 volumes of water R, — as detector a spectrophotometer set at 270 nm.

Salicylic acid • Inject 10 µl of reference solutions (d) and (e). When the chromatograms are recorded in the prescribed conditions, the retention times relative to phenol are: 4 -hydroxybenzoic acid, about 0. 70; 4 hydroxyisophthalic acid, about 0. 90. Adjust the sensitivity of the detector so that the height of the principal peak in the chromatogram obtained with reference solution (f) is at least 70 per cent of the full scale of the recorder. The test is not valid unless: in the chromatogram obtained with reference solution (e), the third peak corresponds to the phenol peak in the chromatogram obtained with reference solution (d) and the resolution between the peaks corresponding to 4 - hydroxyisophthalic acid and to phenol is at least 1. 0. If this resolution is not obtained • adjust the quantity of acetic acid in the mobile phase. Inject 10 µl of the test solution and 10 µl of reference solution (f). In the chromatogram obtained with the test solution: the areas of the peaks due to 4 -hydroxybenzoic acid, 4 -hydroxyisophthalic acid and phenol are not greater than the areas of the corresponding peaks in the chromatogram obtained with reference solution (f) (0. 1 per cent for 4 - hydroxybenzoic acid; 0. 05 per cent for 4 hydroxyisophthalic acid and 0. 02 per cent for phenol).

Salicylic acid
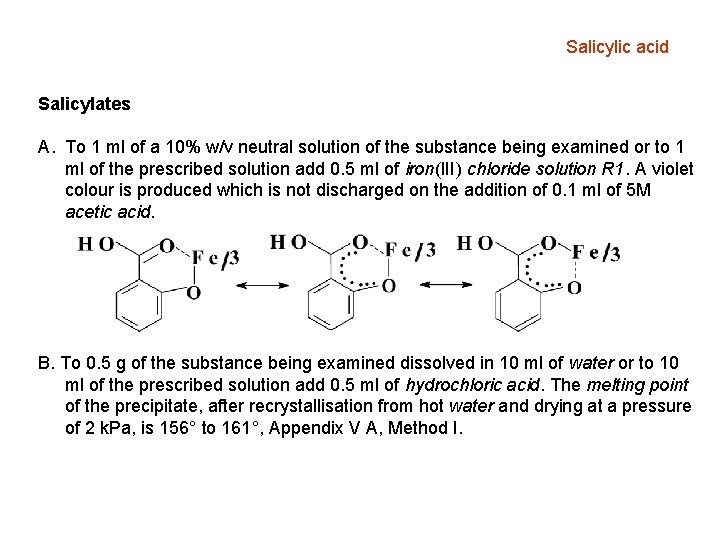
Salicylic acid Salicylates A. To 1 ml of a 10% w/v neutral solution of the substance being examined or to 1 ml of the prescribed solution add 0. 5 ml of iron(III) chloride solution R 1. A violet colour is produced which is not discharged on the addition of 0. 1 ml of 5 M acetic acid. B. To 0. 5 g of the substance being examined dissolved in 10 ml of water or to 10 ml of the prescribed solution add 0. 5 ml of hydrochloric acid. The melting point of the precipitate, after recrystallisation from hot water and drying at a pressure of 2 k. Pa, is 156° to 161°, Appendix V A, Method I.
Salicylic acid
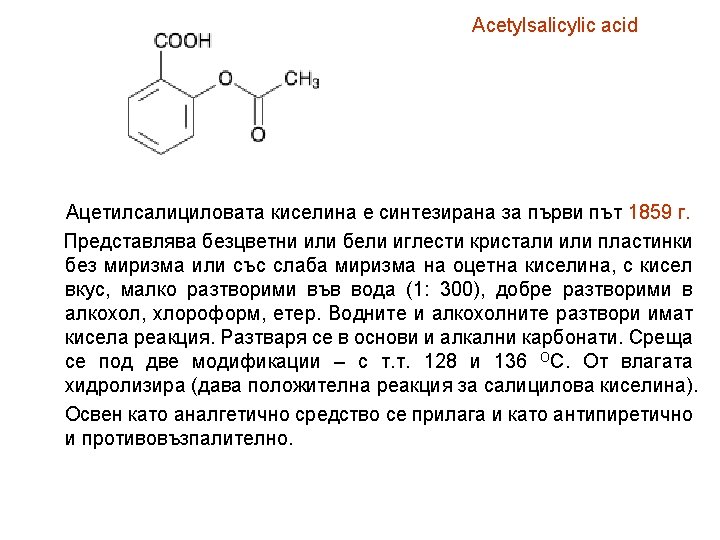

Acetylsalicylic acid

Acetylsalicylic acid Identification A. Examine by infrared absorption spectrophotometry (2. 2. 24), spectrum obtained with acetylsalicylic acid CRS. comparing with the B. To 0. 2 g add 4 ml of dilute sodium hydroxide solution R and boil for 3 min. Cool and add 5 ml of dilute sulphuric acid R. A crystalline precipitate is formed. Filter, wash the precipitate and dry at 100°C to 105°C. The melting point (2. 2. 14) is 156°C to 161°C. C. In a test tube mix 0. 1 g with 0. 5 g of calcium hydroxide R. Heat the mixture and expose to the fumes produced a piece of filter paper impregnated with 0. 05 ml of nitrobenzaldehyde solution R. A greenish-blue or greenish-yellow colour develops on the paper. Moisten the paper with dilute hydrochloric acid R. The colour becomes blue. D. Dissolve with heating about 20 mg of the precipitate obtained in identification test B in 10 ml of water R and cool. The solution gives reaction (a) of salicylates (2. 3. 1).

Acetylsalicylic acid Related substance Examine by liquid chromatography (2. 2. 29). Prepare the solutions immediately before use. Test solution. Dissolve 0. 10 g of the substance to be examined in acetonitrile for chromatography R and dilute to 10. 0 ml with the same solvent. Reference solution (a). Dissolve 50. 0 mg of salicylic acid R in the mobile phase and dilute to 50. 0 ml with the mobile phase. Dilute 1. 0 ml of this solution to 100. 0 with the mobile phase. Reference solution (b). Dissolve 10. 0 mg of salicylic acid R in the mobile phase and dilute to 10. 0 ml with the mobile phase. To 1. 0 ml of this solution add 0. 2 ml of the test solution and dilute to 100. 0 ml with the mobile phase. The chromatographic procedure may be carried out using: — a stainless steel column 0. 25 m long and 4. 6 mm in internal diameter packed with octadecylsilyl silica gel for chromatography R (5 µm), — as mobile phase at a flow rate of 1 ml/min a mixture of 2 volumes of phosphoric acid R, 400 volumes of acetonitrile for chromatography R and 600 volumes of water R, — as detector a spectrophotometer set at 237 nm. Inject 10 µl of each solution. Continue the chromatography of the test solution for seven times the retention time of acetylsalicylic acid. The test is not valid unless in the chromatogram obtained with reference solution (b), the resolution between the two principal peaks is at least 6. 0. In the chromatogram obtained with the test solution the area of any peak, apart from the principal peak, is not greater than the area of the principal peak in the chromatogram obtained with reference solution (a) (0. 1 per cent); the sum of the areas of all the peaks is not greater than 2. 5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0. 25 per cent). Disregard any peak with an area less than 0. 25 times the area of the principal peak in the chromatogram obtained with reference solution (a).
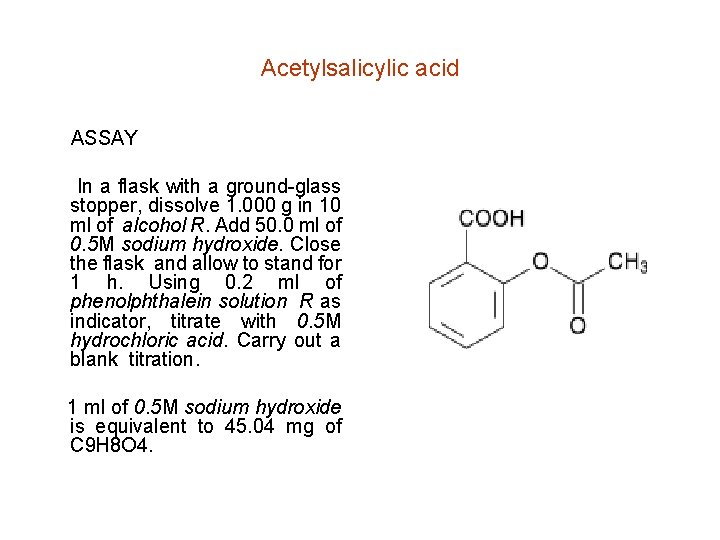
Acetylsalicylic acid ASSAY In a flask with a ground-glass stopper, dissolve 1. 000 g in 10 ml of alcohol R. Add 50. 0 ml of 0. 5 M sodium hydroxide. Close the flask and allow to stand for 1 h. Using 0. 2 ml of phenolphthalein solution R as indicator, titrate with 0. 5 M hydrochloric acid. Carry out a blank titration. 1 ml of 0. 5 M sodium hydroxide is equivalent to 45. 04 mg of C 9 H 8 O 4.
![Acetylsalicylic acid - Impurities D. 2 -[[2(acetyloxy)benzoyl]oxy] benzoic acid (acetylsalicylic acid), A. 4 -hydroxybenzoic Acetylsalicylic acid - Impurities D. 2 -[[2(acetyloxy)benzoyl]oxy] benzoic acid (acetylsalicylic acid), A. 4 -hydroxybenzoic](http://slidetodoc.com/presentation_image_h2/3fc2c744c4cb8b6783df60c13394255c/image-33.jpg)
Acetylsalicylic acid - Impurities D. 2 -[[2(acetyloxy)benzoyl]oxy] benzoic acid (acetylsalicylic acid), A. 4 -hydroxybenzoic acid, E. 2 -[(2 hydroxybenzoyl)oxy] benzoic acid (salicylic acid), B. 4 -hydroxybenzene-1, 3 -dicarboxylic acid (4 -hydroxyisophthalic acid), C. 2 -hydroxybenzoic acid (salicylic acid), F. 2 -(acetyloxy)benzoic anhydride (acetylsalicylic anhydride). Ph Eur

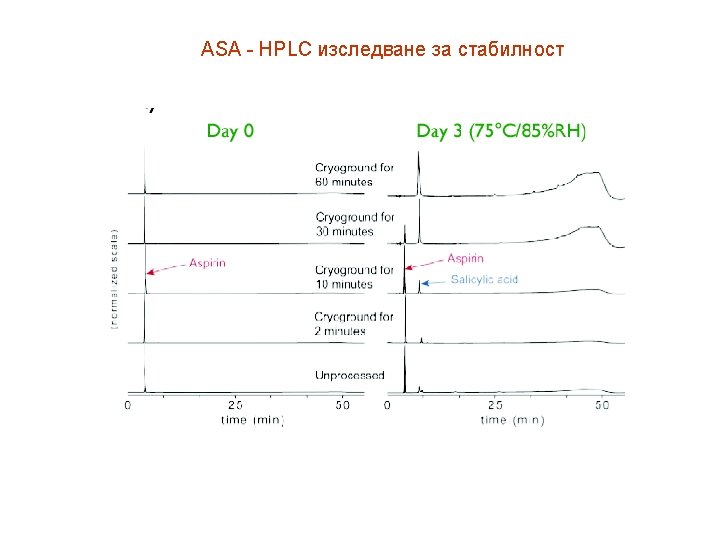
ASA Determination of free salicylic acid in chewing aspirin tablets by HPLC. Tian J, Chen XS, Wang RD. Department of Pharmacy, General Navy Hospital of PLA, Beijing 100037, China. OBJECTIVE: To establish a HPLC method for determining the content of free salicylic acid in chewing aspirin tablets. METHOD: The determination was conducted on a HPLC column (C(18), 150 mm x 4. 6 mm x 5 microm) with methanolwater-glacial acetic acid (8. 0 5. 5 1. 0) as the mobile phase and the detection wavelength of 302 nm. RESULTS: The calibration curve was linear within the concentration range of 2. 65 to 31. 77 microg/ml (r=0. 99997) of salicylic acid. The average recovery rate was 100. 21% with relative standard deviation of 0. 53% (n=6). CONCLUSION: HPLC is quick and accurate of determining the content of free salicylic acid for chewing aspirin tablets.

ASA Caffeine and Aspirin by HPLC/ELSD Separation of caffeine and aspirin was achieved by reversed phase isocratic elution with water/methanol/acetic acid in around 2 minutes using an Econosphere C 18, 3µm, 30 x 4. 6 mm column (Part No. 28213). Detection was by evaporative light scattering using an Alltech 500 ELSD (Evaporative Light Scattering Detector). In ELSD, the mobile phase is first evaporated. Solid particles remaining from the sample are then carried in the form of a mist into a cell where they are detected by a laser.
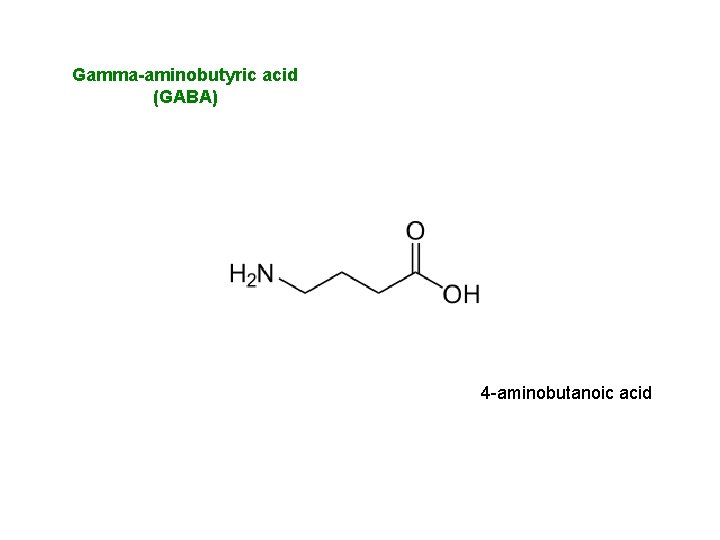
Gamma-aminobutyric acid (GABA) 4 -aminobutanoic acid
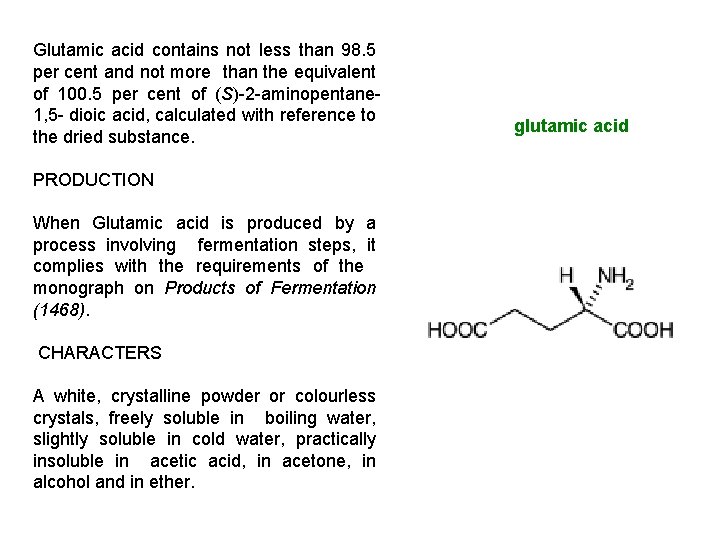
Glutamic acid contains not less than 98. 5 per cent and not more than the equivalent of 100. 5 per cent of (S)-2 -aminopentane 1, 5 - dioic acid, calculated with reference to the dried substance. PRODUCTION When Glutamic acid is produced by a process involving fermentation steps, it complies with the requirements of the monograph on Products of Fermentation (1468). CHARACTERS A white, crystalline powder or colourless crystals, freely soluble in boiling water, slightly soluble in cold water, practically insoluble in acetic acid, in acetone, in alcohol and in ether. glutamic acid

glutamic acid Ninhydrin-positive substances Examine by thin-layer chromatography (2. 2. 27), using a TLC silica gel plate R. Test solution (a). Dissolve 0. 10 g of the substance to be examined in 5 ml of dilute ammonia R 2 and dilute to 10 ml with water R. Test solution (b). Dilute 1 ml of test solution (a) to 50 ml with water R. Reference solution (a). Dissolve 10 mg of glutamic acid CRS in water R and dilute to 50 ml with the same solvent. Reference solution (b). Dilute 5 ml of test solution (b) to 20 ml with water R. Reference solution (c). Dissolve 10 mg of glutamic acid CRS and 10 mg of aspartic acid CRS in water R and dilute to 25 ml with the same solvent. Apply separately to the plate 5 µl of each solution. Dry the plate in a current of air for 15 min. Develop over a path of 15 cm using a mixture of 20 volumes of glacial acetic acid R, 20 volumes of water R and 60 volumes of butanol R. Allow the plate to dry in air, spray with ninhydrin solution R and heat at 100°C to 105°C for 15 min. Any spot in the chromatogram obtained with test solution (a), apart from the principal spot, is not more intense than the spot in the chromatogram obtained with reference solution (b) (0. 5 per cent). The test is not valid unless the chromatogram obtained with reference solution (c) shows two clearly separated spots.
Acid-base extraction is a procedure using sequential liquid-liquid extractions to purify acids and bases from mixtures based on their chemical properties. Acidbase extraction is routinely performed during the work-up after chemical syntheses and for the isolation of compounds and natural products. The product is largely free of neutral and acidic or basic impurities. It is not possible to separate chemically similar acids or bases using this simple method. Alternatives to acid-base extraction including: - filtering the mixture through a plug of silica gel or alumina — charged salts tend to remain strongly adsorbed to the silica gel or alumina - ion exchange chromatography can separate acids, bases, or mixtures of strong and weak acids and bases by their varying affinities to the column medium at different p. H.

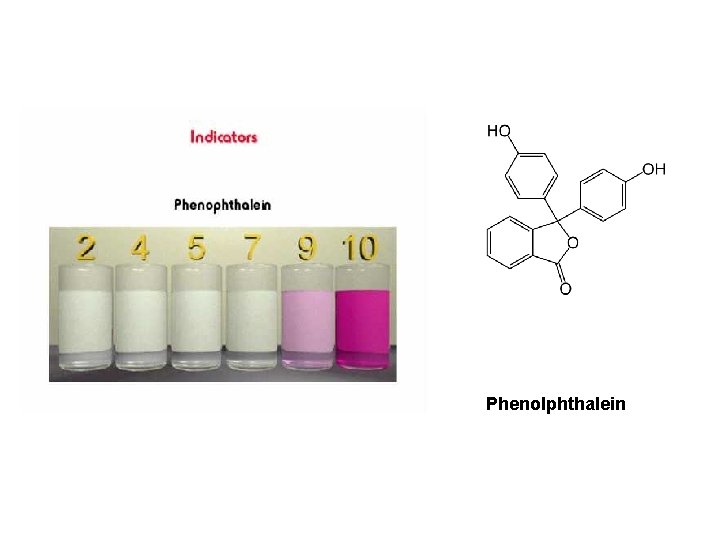
Phenolphthalein







- Slides: 50